, Mark Thomas1 and David Milford2
(1)
Department of Renal Medicine, Birmingham Heartlands Hospital, Birmingham, UK
(2)
Birmingham Children’s Hospital, Birmingham, UK
Abstract
In this chapter we explain:
How ACE inhibitors and angiotensin receptor blockers work
How drugs can be nephrotoxic through
Vasoconstriction
Tubulotoxicity
Interstitial inflammation
MINT cocktails
The interaction between metformin and kidney function
Kidney damage due to heroin, cocaine, mushrooms and chemicals
A detailed drug history is a crucial part of the assessment of any patient, especially when kidney disease is suspected. The history must cover the nature of any drugs taken – prescribed or otherwise. The use of alternative remedies derived from plants and animals is increasing worldwide and many are associated with acute kidney injury [1].
Details of each drug, when it was started and stopped, and the doses prescribed and taken should be compared to changes in the patient’s kidney function.
Some commonly used drugs can affect kidney function and/or structure, in other words can be nephrotoxic. There are three main mechanisms of injury: ischaemia, tubular toxicity and interstitial inflammation. Injury to the glomerulus is less common [2, 3].
Vasoactive Drugs
For glomerular filtration to occur, blood needs to flow into the glomeruli at an adequate rate and under sufficient pressure to force filtration through the glomerular filtration barrier (see Sect. “Turning blood into urine” in page 1). Vasoactive drugs can affect glomerular filtration by varying vascular resistance and the dynamics of glomerular blood flow.
ACE Inhibitors and Angiotensin Receptor Blockers
Angiotensin-II (A-II) constricts the efferent arterioles , increasing the glomerular filtration pressure by restricting the flow of blood out of the glomeruli. The effect of A-II is inhibited by Angiotensin Converting Enzyme (ACE) inhibitors , which block the synthesis of A-II, and Angiotensin Receptor Blockers (ARBs) , which inhibit the action of A-II on its receptor (Fig. 8.1). Both classes of drug allow the efferent arterioles to dilate and so lower the glomerular filtration pressure. The drop in GFR is usually small and the reduction in intraglomerular pressure can be beneficial by reducing hydrostatic damage and glomerulosclerosis.
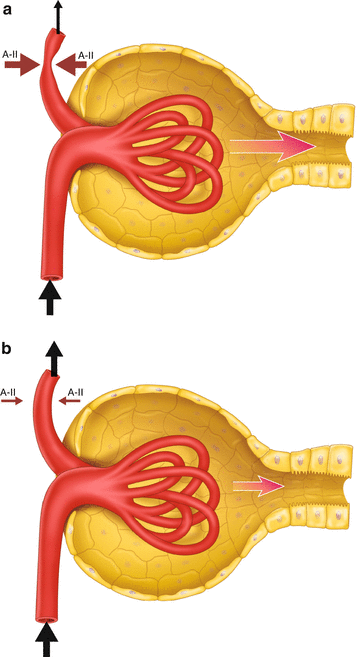

Fig. 8.1
(a) The effect of angiotensin II on glomerular filtration. AII causes vasoconstriction of the efferent arteriole and increases glomerular filtration pressure and rate. (b) Inhibition of AII leads to dilatation of the efferent arteriole, releasing the intra-glomerular pressure and reducing glomerular filtration rate
If the pressure in the afferent arteriole is low, glomerular filtration is dependent upon vasoconstriction of the efferent arteriole to maintain intraglomerular pressure. This is the case in someone with poor left ventricular function and low systolic blood pressure or with atherosclerosis of the renal arteries causing a drop in pressure upstream of the glomeruli.
If such a person is given an ACEI or an ARB, there is a risk that the drop in intraglomerular pressure will be clinically significant (Patient 8.1). There is a typical pattern of changes in the laboratory results that helps to identify this effect:
↓ eGFR – low glomerular filtration pressure
↑ potassium – reduced AII stimulation of aldosterone production, leading to reduced potassium excretion
↑ urea-to-creatinine ratio – slow flow of filtrate along the nephron allowing diffusion of urea back into the blood (see Sect. “Serum urea and creatinine – different measures of kidney function” in page 26).
Patient 8.1: Recurrent ACE Inhibitor Nephrotoxicity
Mrs. Blake, aged 72 years, had high blood pressure, proteinuria and diabetes. She was started on the ACE inhibitor lisinopril and her blood pressure became well controlled. Over the following year her eGFR steadily declined and the serum urea and potassium levels rose, suggesting that the ACE inhibitor was the cause (Fig. 8.2). The lisinopril was stopped and her eGFR returned to its previous level. The high blood pressure was controlled with a calcium channel blocker.
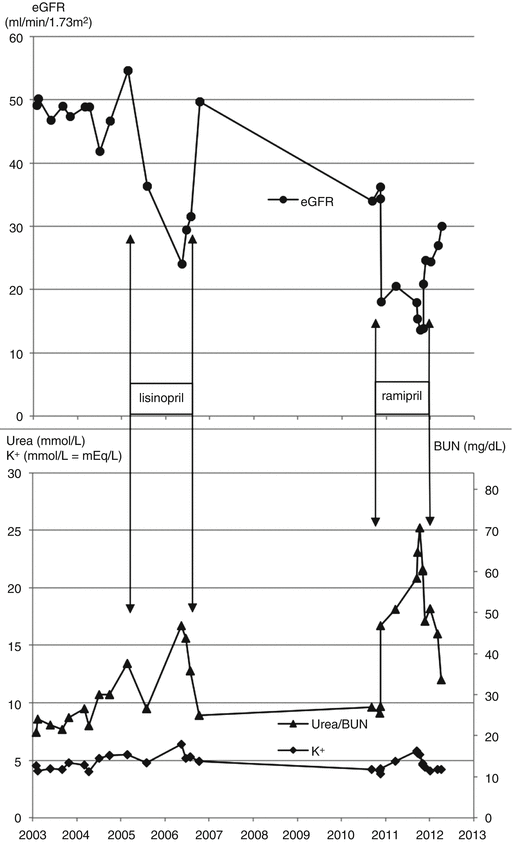

Fig. 8.2
Changes in eGFR, serum urea and serum potassium during repeated periods of ACE inhibitor treatment
Four years later, a doctor unfamiliar with her history reviewed Mrs Blake in the diabetes clinic. This doctor was concerned about the heavy proteinuria and so started ramipril.
Changes in eGFR, urea and potassium repeated the pattern of 4 years previously, this time to more extreme values as her underlying kidney function had worsened (Fig. 8.2).
Non-steroidal Anti-inflammatory Drugs
Blood flow through kidney arterioles is maintained by vasodilator prostaglandins . Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit cyclo-oxygenase enzymes and reduce the production of prostaglandins. Removing the vasodilator effect of prostaglandins leads to vasoconstriction and a drop in renal blood flow and GFR.
Overdosage of NSAIDs can cause acute kidney injury (Patient 8.2). Normal doses given repeatedly over a prolonged period can lead to permanent damage from ischaemia.
Patient 8.2: NSAID Overdose
John, an 18-year-old musician who suffered from depression, was admitted having taken an overdose of 300 mg seroxat, 700 mg diclofenac and 3.5 g naproxen. His U&E’s were normal: creatinine = 103 micromols/L (1.2 mg/dL), eGFR = 87. After a psychiatric assessment, he went home.
Over the next 2 days he became unwell with vomiting and pain in the loins and so returned to hospital. His eGFR had dropped to 50 and continued to decline over the following 3 days (Fig. 8.3). In retrospect, the initial creatinine result had indicated acute kidney injury as 2 years previously it had been 77 micromols/L (0.9 mg/dL). The peak serum creatinine was 287 micromols/L (3.2 mg/dL) making this episode AKI stage 3 (see Table 3.2).
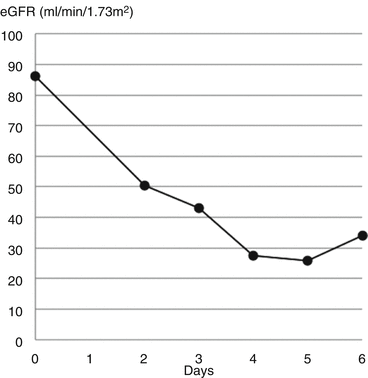

Fig. 8.3
Changes in eGFR with time after overdose of NSAIDs
Calcineurin Inhibitors – Two Sides of a Coin
Calcineurin inhibitors such ciclosporin and tacrolimus preserve the function of kidney transplant s by preventing rejection. Ironically, one of their main side effects is to reduce GFR by vasoconstriction of kidney arterioles. With chronic use, this leads to ischaemic damage and fibrosis , arranged in stripes along the radial distribution of the intrarenal arterioles (Fig. 8.4).
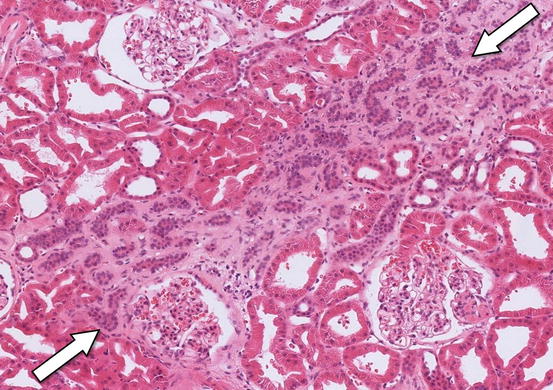

Fig. 8.4
A transplant kidney biopsy showing a band of fibrosis through the interstitium caused by long-term ciclosporin therapy (arrows). The glomeruli are normal. Haematoxylin and eosin ×200
Mixing Vasoactive Drugs to Make MINT Cocktails
The commonest situation causing acute kidney injury is where a number of drugs that alone would not significantly reduce GFR are used together. This has been termed a Multifactorial Iatrogenic NephroToxicity or MINT cocktail .1
In patients with diabetes , long-standing hypertension, vascular disease or advanced age, kidney blood supply may be chronically compromised. Combining an ACE inhibitor , which reduces intraglomerular pressure, and a diuretic , which drops plasma volume and thereby renal blood flow, can lead to a marked drop in GFR (see Patient 8.1). Patients with normal kidneys can suffer acute kidney injury if a sufficient number of nephrotoxic factors are combined (see Patient 8.3).
Patient 8.3: A MINT Cocktail
Mrs. Kent was admitted as an emergency on 31st October having had a painful swollen right knee for 3 weeks. She had had no fever but her CRP (C-reactive protein) was very high, 237 mg/L, suggesting a septic arthritis. Her urine contained + protein on dipstick testing. Her serum creatinine was 57 micromols/L (0.6 mg/dL).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








