Established
Probable
Possible in selected children
Rotavirus
Torovirus
HIV
Norovirus
Aichi virus enterovirus 22
Cytomegalovirus
Adenovirus
Epstein–Barr virus picobirnavirus
Astrovirus
The viruses most frequently responsible for acute gastroenteritis in children belong to four distinct families: rotaviruses, caliciviruses, astroviruses, and enteric adenoviruses. Rotavirus and norovirus are the two leading agents of acute diarrhea. Other viruses, such as toroviruses, picornaviruses (the Aichi virus), and enterovirus 22, play a minor epidemiological role. Finally, selected viruses induce diarrhea only in children at risk. These include cytomegalovirus, Epstein–Barr virus, picobirnaviruses, and HIV.
Pathophysiology of Viral Diarrhea
In the classic and simple view, the pathogenesis of diarrhea may be divided into osmotic and secretory. Viral diarrhea was originally believed to be the consequence of endoluminal fluid accumulation osmotically driven by non-absorbed nutrients due to cell invasion and epithelial destruction by enteropathogenic agents. It is now known that several mechanisms are responsible for diarrhea, depending on the specific agents and the host features. In addition, selected viruses have multiple virulence pathways that act synergistically to induce diarrhea.
The mechanisms of diarrhea induced by group A rotaviruses have been extensively investigated and provide a paradigm of the pathophysiology of viral diarrhea [20–22]. Rotavirus has both a tissue- and a cell-specific tropisms, and it infects the mature enterocyte of the small intestine. The first step is virus binding to specific receptors located on the cell surface, the GM1 ganglioside. However, different rotavirus strains bind in either a sialic-acid-dependent or sialic-acid-independent fashion. Most rotaviruses, including all human strains, infect polarized enterocytes through both the apical and the basolateral side, in a sialic-acid-independent manner, suggesting the presence of different receptors. The early stages of rotavirus binding involve the viral protein (VP4) spike attachment and cleavage. After binding, rotavirus enters into the cell by a multistep process that requires both VP7 and VP4 proteins. Infection of the villous enterocytes leads to cell lysis, compromising nutrient absorption and driving fluid into the intestinal lumen through an osmotic mechanism. However, the destruction of villus-tip cells induces a compensatory proliferation of crypt cells. These immature enterocytes physiologically maintain a secretive tone, thus contributing to diarrhea with ion secretion, as the result of the imbalance between absorptive villous and secretory crypt cells. Thus, the cytopathic effect of rotavirus results in both osmotic and secretory diarrhea . Histological changes induced by rotavirus infection occur within 24 h of infection in animal models .
The enteric nervous system may also play a direct role in inducing fluid secretion, similar to that induced by cholera toxin and other intestinal secretagogues. The molecular mechanisms of fluid secretion have also been investigated. Rotavirus induces an increase in intracellular calcium levels, which is responsible for the disassembly of microvillar F-actin, the perturbation of cellular protein trafficking, the damage of tight junction, with a disruption of cell–cell interaction and cytolysis .
In children with rotavirus infection, the onset of diarrhea is abrupt and occurs in the absence of histological changes, suggesting that in the initial phase of the infection a secretory pathway is responsible for diarrhea. The identification of the nonstructural protein (NSP4), an enterotoxin produced by rotavirus, responsible for fluid secretion but not for epithelial damage may explain this phenomenon. NSP4 is a multifunctional virulence factor (VF), as it possesses the following features (Fig. 14.1): It is released from infected cells and enters the cells through a specific receptor causing calcium-dependent chloride secretion. NSP4 also alters plasma membrane permeability and is cytotoxic. NSP4 is the only rotavirus gene product capable of eliciting intracellular calcium mobilization. NSP4 further contributes to diarrheal pathogenesis by directly altering enterocyte actin distribution and paracellular permeability. Finally, NSP4 plays a role in the inhibition of the Na+-dependent glucose transporter (SGLT-1). Glucose absorption as well as disaccharidase activities are impaired in rotavirus enteritis, whereas the Na/amino acid co-transporters are not involved .
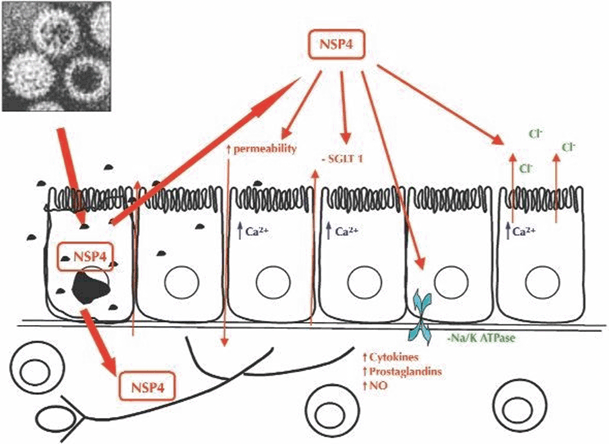

Fig. 14.1
Combined effects by NSP4 in the pathophysiology of rotavirus diarrhea. Rotavirus infects epithelial cells of the small intestine, replicates, and induces cell lysis. NSP4 is released by infected cells and functions as a Ca2+-dependent enterotoxin triggering Cl− secretion. It decreases fluid and electrolyte transport by inhibiting Na–glucose symport SGLT1 and, possibly Na–K adenosine triphosphatase (ATPase). It also impairs disaccharidase expression. Furthermore, rotavirus and/or NSP4 may diffuse underneath the intestinal epithelium activating secretory reflexes in the enteric nervous system. Late during the infection, an inflammatory response in the lamina propria may be detected, and the production of inflammatory substances and cytokines may further contribute to the increase of intestinal permeability and diarrhea. NSP4 nonstructural protein, SGLT-1 Na+-dependent glucose transporter, NO nitrous oxide. (Reprinted with permission from Ref. [19], 2004, Fig. 9.3, p. 131)
Rotavirus diarrhea may also have an inflammatory component. The induction of cytokines is important in developing an inflammatory and immune response, especially in intestinal infection caused by bacteria. In rotavirus infection, limited inflammation is detected by histological studies, suggesting that cytokines are effective in inducing a host immune response to rotavirus diarrhea. However, it has been shown that rotavirus-infected enterocytes activate nuclear factor kappa B (NF-κB) and the production of chemokines interleukin (IL)-8, Rantes, and growth related oncogene (GRO)-a, of interferon (IFN)-α, and of granulocyte/macrophage–colony-stimulating factor (GM-CSF). Recent evidence suggests that rotavirus-induced diarrhea may be also associated with an increase of intestinal motility through the stimulation of myenteric nerve plexus [23].
In conclusion, the primary target of rotavirus is the enterocyte, which is induced to secrete fluids and electrolytes and is subsequently destroyed. On the other hand, the enterocyte acts as a sensor to the mucosa with the production of viral and endogenous factors and the activation of other cell types including neurons. Thus, rotavirus-induced diarrhea is a multistep and multifactorial event, in which fluid secretion and cell damage are observed in a precise sequence, as shown in an intestinal cell line-based experimental model [24] (Fig. 14.2). A summary of the multiple mechanisms involved in the rotavirus–intestine interaction is provided in Table 14.2 .
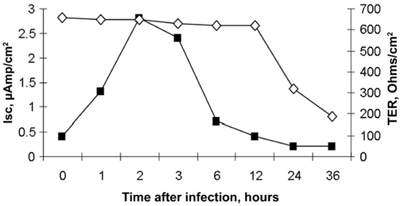

Fig. 14.2
Biphasic effect of rotavirus in Caco-2 cells. Rotavirus induces a biphasic response, in an in vitro model of infection in Caco-2 enterocytes mounted in Ussing chambers. An early secretion is evident in the first few hours of infection, with a peak at 2-h postinfection, as shown by the increase in short circuit current (Isc, µA). Subsequently, rotavirus exerts a cytotoxic effect with a loss of tissue integrity, as demonstrated by the fall of transepithelial resistance (TER) (Ohm/cm2) which is evident at 24-h postinfection. The results suggest that rotavirus diarrhea is initially the result of an early secretory mechanism and of a subsequent osmotic pathway, due to cell damage and loss of functional absorptive surface, leading to nutrient malabsorption. (Reprinted with permission from Ref. [19]. Reprinted from Ref. [24], by Permission of Oxford University Press)
Table 14.2
Mechanisms involved in rotavirus-induced diarrhea
Mechanism | Effect |
|---|---|
Enterocyte damage | Nutrient malabsorption/osmotic diarrhea |
Crypt cell proliferation | Ion and water secretion/secretory diarrhea |
NSP4 production | Increase in intracellular calcium, chloride secretion/secretory diarrhea |
NSP4 inhibition of SGLT-1 | Glucose malabsorption/osmotic diarrhea |
Neuromediated vascular ischemia | Secretory diarrhea induced by neurotransmitter release |
Inflammation | NF-kB, IL-8, Rantes release/osmotic, and secretory diarrhea |
Stimulation of myenteric nerve plexus | Increase in intestinal motility |
Clinical Signs and Symptoms of Viral Diarrhea
Usually viral diarrhea has an abrupt onset and lasts for 3–5 days. Generally it is a benign condition with spontaneous recovery. In very select cases, diarrhea may be persistent lasting more than 7–15 days. Risk factors for persistent diarrhea in children are: young age, early weaning, malnutrition, and immunodeficiency. In selected children, an acute onset may coincide with the first manifestation of celiac disease .
The predominant symptoms of acute gastroenteritis, regardless of the causative etiologic agent, are diarrhea and vomiting, associated or not to fever and abdominal pain . None of the symptoms that usually characterize the clinical feature of acute gastroenteritis has a strong predictive value able to discriminate among the different etiologies. However, there are selected features that may help a differential diagnosis of viral versus bacterial infection [25] .
Children with viral intestinal infection generally have large volumes of watery stools, suggesting small bowel involvement, and vomiting and fever are more frequent than in bacterial diarrhea. Each of these features contributes to dehydration. Another feature of viral gastroenteritis is the frequent association of diarrhea and vomiting with respiratory symptoms (Table 14.3).
Table 14.3
Clinical features associated with viral and bacterial agents of acute diarrhea in children
Viral diarrhea | Bacterial diarrhea |
|---|---|
Watery diarrhea | High fever |
High volume stools | Bloody stools |
Vomiting | Dysentery |
Fever | Abdominal pain |
Presence of respiratory symptoms | Neurological signs |
In contrast, the presence of symptoms suggesting colitis, such as a high number of diarrheal episodes with small amount of stools, blood in the stools, high fever, and abdominal pain is more likely associated with bacterial etiology (Table 14.3) [26, 27].
The severity of acute gastroenteritis is reflected by the degree of dehydration. The degree of dehydration should be evaluated at first observation and during the follow-up to establish the efficacy of rehydration treatment. However, high persistent fever and lethargy may be signs of a more severe clinical condition and indicate systemic involvement. Moreover, benign seizures, not related with electrolyte imbalances, have been reported for rotavirus- and norovirus -induced gastroenteritis [28, 29]. Severe encephalopathies were reported in a recent surveillance study conducted in Germany on about 100 cases of very severe diarrhea [30] .
When compared with other viral infections, rotavirus infection is more frequently associated with high-grade fever (> 38 °C), frequent diarrheal episodes (> 7/day), and long-lasting diarrhea that results in significantly higher severity scores [31–36]. In contrast, children with norovirus infection have significantly more episodes of vomiting than other viral infections, and in some cases, vomiting may be the only gastrointestinal symptom and up to 20 % of children are present without diarrhea [33, 34]. Intestinal infections due to adenovirus, on the other hand, seem to have milder clinical features [36] .
Diagnosis
Most children with acute diarrhea have viral gastroenteritis. Microbiological examination is not helpful in the majority of cases and should be reserved for special circumstances. In fact, regardless of etiology, most children do not require any etiology-based treatment and therefore identification of a specific pathogen is not generally needed. Microbiological investigation should however be performed during outbreaks, especially in childcare settings, schools, hospitals, or residential settings to identify the pathogen and establish its source in the attempt to reduce transmission. Stool samples should also be taken from children with bloody diarrhea, a history of recent foreign travel, and from young or immunocompromised children with high fever for whom antibiotic treatment is considered. Finally, it is also recommended to investigate children in whom diarrhea persists for more than 10–14 days, or when a noninfectious etiology for diarrhea is suspected, such as inflammatory bowel disease (IBD) (Table 14.4) [25]. Several techniques are available to identify the specific etiology of viral diarrhea. The gold standard is viral culture but its clinical application is limited, due to the costs, the delay in the results and the complexity of the procedures. Immunofluorescence or latex agglutination is widely used to identify fecal viruses. Polymerase chain reaction (PCR) is becoming a common diagnostic tool for virus identification. Specific PCR are currently available for norovirus , rotavirus, adenovirus, cytomegalovirus, and other less common viruses .
Table 14.4
Indications to microbiological evaluation in children with acute diarrhea
Condition |
|---|
Age < 3 months |
Shock or septic appearance |
> 10 liquid stools/day, high fever, dysentery, bloody stools |
Recent history of travel |
Immunocompromised children |
Outbreak |
Protracted or chronic diarrhea |
Differential diagnosis of viral gastroenteritis may include food poisoning, which is eventually indirectly related to a microbial etiology. Food poisoning has a more rapid onset, often with vomiting and is more common in children than in infants, being related to ingestion of at risk foods. On the other hand, when diarrhea is persistent, lasting more than 7 days, a different etiology should be considered [37]. Common causes of persistent diarrhea (dealt with in detail in Chap. 17) include small intestinal bacterial overgrowth , lactose intolerance, and cow’s milk protein intolerance; it should, in addition, be considered that chronic inflammatory conditions of the small and/or large intestine such as celiac disease or IBD may have an acute onset or be triggered by viral enteritides .
Specific Viruses
Rotavirus
Rotavirus is a double-stranded RNA virus belonging to the Reoviridae family. The virion, 70–75 nm, is composed of a three-layered protein capsid that encloses 11 distinct segments of genomic RNA, each coding for a different capsid or nonstructural protein. The internal core contains VP 1, 2, and 3; the inner capsid contains VP4; the two outer capsid proteins encoded by genes 4 and 7, namely VP4 and VP7, represent the only established neutralization antigens of the virus. The protective role of antibodies directed against these proteins has been confirmed in both experimental animal models and humans. A possible role has been suggested for antibodies directed at the inner capsid protein VP6, which is not associated with in vitro neutralization. The nonstructural proteins NSP1, NSP2, and NSP4 are VFs in mice. Rotavirus groups A–F have been described, but only groups A, B, and C have been identified in humans. Most human infections are caused by group A rotaviruses that are classified into serotypes by a dual classification system based on neutralizing antigens on two outer capsid proteins, VP7 (G serotype) and VP4 (P serotype). To date, 14 G serotypes and 11 P serotypes have been identified in humans. There is substantial genetic diversity within each G and P type. Predominant serotypes vary from year to year and from region to region. G1P [8] is the globally predominant strain, representing over 70 % of rotavirus infections in North America, Europe, and Australia. G9 strains now constitute the predominant strains in some parts of Asia and Africa, and G8 strains are proportionally more frequent in Africa [38, 39]. In South America, G5 strains have emerged in children with diarrhea, and G9 is associated with more severe disease in Latin America [40]. Similarly, the distribution of P [6] antigen differs according to region. An example is the rapidly evolving change of serotype distribution in Africa [41] .
Specific strains may express stronger VFs, which could be related to the severity of symptoms. More severe presentations may also be related to the reintroduction of strains in areas where they have been previously absent. The epidemiology of rotavirus shows a link with cold seasons with a higher incidence during fall and winter.
Transmission is by fecal–oral route, both through close person-to-person contact and by fomites. Viruses are shed in high concentrations in the stool of rotavirus-infected persons. Children shed large numbers of viruses in stool, during the acute illness but they may shed rotavirus 2 days before and up to 10 days after the onset of symptoms. The virus may also be transmitted by respiratory droplets. Spread within families, institutions, hospitals , and childcare settings is common. Rotavirus is a major cause of acute gastroenteritis in children attending child care. Rotavirus is also responsible for nosocomial infection during the winter with an incidence as higher as 3 % hospitalization in a meta-analysis of 20 studies in Europe and North America [42], prolonging hospital stays and increasing medical costs. The incidence of nosocomial infection is directly related to the duration of hospital stay. In Italy, the incidence of nosocomial rotavirus -induced gastroenteritis was 7.9/1000 child-days of hospitalization [43].
The incubation period for rotavirus diarrhea is short, usually less than 48 h. Rotavirus is able to determine a large spectrum of disease, ranging from asymptomatic shedding to severe dehydration, seizures, and even death. Rotavirus gastroenteritis typically begins with acute onset of fever and vomiting followed 24–48 h later by watery diarrhea [44]. Symptoms generally persist for 3–8 days, although protracted episodes have been reported occasionally. Fever is usually of low grade and occurs in up to half of all infected children. Vomiting occurs in 80–90 % of infected children, and it is usually brief, lasting 24 h or less in most children. Dehydration and electrolyte disturbances are the major complications of rotavirus infection and occur most often in the youngest children. Studies of hospitalized children have indicated that gastroenteritis associated with rotavirus is more severe than cases in which rotavirus was not detected, with more severe dehydration, higher incidences of vomiting and higher needing of parenteral rehydration. Children with immunodeficiency, particularly those with T cell immunodeficiencies or severe combined immunodeficiency (SCID), and children after bone marrow transplantation are at higher risk of severe and prolonged diarrhea and central nervous system complications.
Diagnosis of rotavirus infection is performed using enzyme immunoassays and latex agglutination assays for detection of group A rotavirus antigen in stools. Virus can also be identified in stool by electron microscopy and by reverse transcriptase-PCR.
Norovirus
Noroviruses are single-stranded RNA viruses belonging to the family Caliciviridae. The prototype virus of the noroviruses, Norwalk virus, was identified in 1972. The availability of molecular diagnostic methods based on reverse transcription-PCR (RT-PCR) highlighted the etiologic role of norovirus in epidemic and sporadic gastroenteritis [45, 46]. Norovirus is now a well-documented leading agent of epidemic gastroenteritis in all age groups, causing > 90 % of nonbacterial and ≈ 50 % of all-cause epidemic gastroenteritis worldwide [47]. The impact of norovirus disease may be much greater than previously thought, and the disease may be more severe in some populations [46, 48–50].
The norovirus genome comprises a single-stranded, positive sense, polyadenylated RNA of approximately 7.5 kb in length, encompassing three open reading frames (ORFs 1–3).
Noroviruses encompass five distinct genogroups (GI-GV) with GI, GII, and GIV infecting humans. The norovirus genogroups are further divided into genotypes and variants (subgenotypes) based on the sequence diversity [51] .
In contrast to rotavirus, the mechanisms of norovirus-induced diarrhea are not well defined. Many viral factors can interfere with basic cellular functions. Several observations showed that, in infected mucosa, villus length was decreased by 25 %, whereas crypt length was unchanged [21]. The villus blunting and the shortening of the villi observed in this viral infection depend on the norovirus infection of intestinal epithelial cells located on the apical area of villi. The infected cells show an increase in cell death rates with a reduction of the overall absorptive surface [52].
Evaluations of human intestinal biopsies from norovirus-infected patients in Ussing chambers showed an active Cl− secretion, consistent with cystic fibrosis transmembrane conductance regulator (CFTR) activation. A reduction of occludin expression as well as claudins 4 and 5 corresponding with a marked decrease in transepithelial resistance has also been observed [53]. In addition, norovirus produces two VP, p48 and p20. The former interferes with cell proteins involved in the regulation of vesicle trafficking [54], whereas p20 impairs actin cytoskeleton structure leading to intestinal barrier dysfunction [55] .
Intestinal mucosal immunity is also affected by norovirus. A recent study showed that dendritic cells were depleted in norovirus-infected mucosa associated with an altered antibody response. On the contrary, dendritic cells were required for a dissemination of the virus to secondary lymphoid tissues supporting the idea that enteric viruses can use dendritic cells to facilitate their dissemination within the host [56]. In addition, the VF1 expression enables norovirus to establish efficient infection interfering with interferon-mediated response pathways and apoptosis [57, 58]. Finally, Nelson et al. observed a highly altered gut microbiota in patients with norovirus gastroenteritis resulting in a low grade of diversity and increased proteobacteria [59]. Elevated proteobacteria is a common feature in patients with dysbiosis, and a reduction in the diversity is associated with several altered functions of gut microbiota [60] .
Norovirus gastroenteritis may occur in three distinct epidemiological settings, and is associated with a broad spectrum of clinical outcomes. Firstly, food-borne gastroenteritis generally affects large numbers of healthy adults over a short time period, with symptoms typically resolving within 1–3 days [61]. Secondly, health-care-associated infection, which occurs in semi-closed settings such as hospital wards and residential/care homes, can be very challenging to contain. Elderly and compromised individuals are frequently affected and are at high risk of prolonged clinical course, typically 4–6 days, with a not negligible mortality rate [62]. Thirdly, norovirus gastroenteritis may also occur sporadically in children, where norovirus is the second most common cause of acute viral gastroenteritis after rotavirus, often requiring hospitalization [63].
Several prospective clinical studies in different geographical areas have demonstrated that norovirus is the second most frequent pathogen after rotavirus in children hospitalized for acute gastroenteritis and is more prevalent in winter. Compared with rotavirus enteritis, the duration of vomiting and diarrhea is significantly longer but norovirus infection is slightly less severe in terms of severity score and need of intravenous (IV) rehydration compared to rotavirus infection [64–66] .
Evaluation and Treatment of Children with Acute Diarrhea
The initial clinical approach to the child with acute gastroenteritis is clinical evaluation . Hydration status in children should be assessed based on easily observed signs and symptoms. Dehydration should be estimated using a score system. An easy to use and reliable score system to evaluate dehydration in children is the Clinical Dehydration Scale (CDS) [67]. It consists of four clinical items: general appearance, eyes, mucous membranes, and tears each of which is scored 0, 1, or 2 for a total score of 0–8 (Fig. 14.3). Severe dehydration is an indication for hospital admission and for IV rehydration.
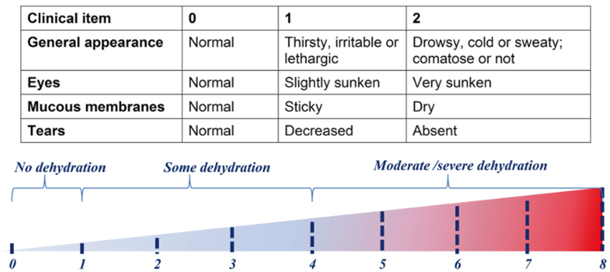





Fig. 14.3
Clinical dehydration scale for children with acute diarrhea. (Modified with permission from Wiley for Ref. [67]. © 2010 by the Society for Academic Emergency Medicine with permission from Wiley)
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







