circumferentially in the bowel wall. This plexus is drained by short veins, which penetrate the external muscle layers, chiefly along the mesenteric margin and then pass to branches of the superior mesenteric vein in the mesentery. The superior mesenteric vein receives venous drainage of the distal duodenum, the jejunum, the ileum, the appendix and cecum, the ascending and transverse colons and a right gastroepiploic vein draining the stomach, before joining the splenic vein to form the hepatic portal vein.13, 15, 18
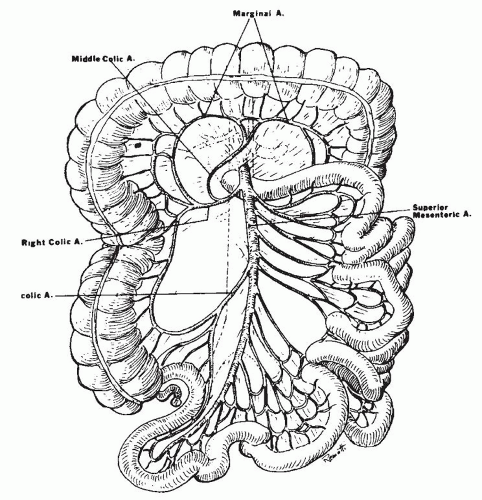
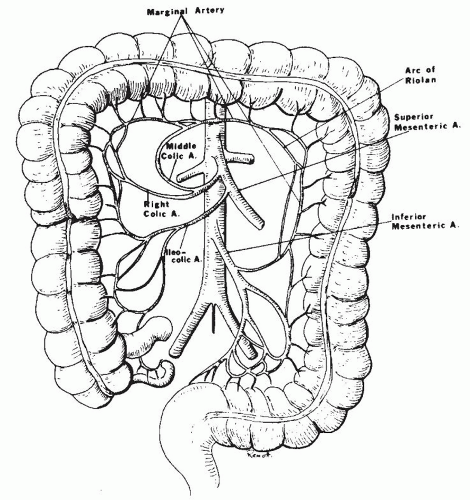
the body of the pancreas (Fig. 2-2). It emerges from under the lower border of the pancreas, passes forward anteriorly over the upper border of the third portion of the duodenum, and descends anteriorly into the mesentery. Usually, the middle colic artery arises from the superior mesenteric artery just before it enters the mesentery. The middle colic artery can arise as a separate branch or be derived from a common right colic-middle colic trunk (53% of the cases). Occasionally, it arises directly from the celiac artery. When the middle colic artery has a large branch running parallel and posterior to it in the transverse mesocolon, this branch is often described as the arc of Riolan. The right colic artery can arise directly (38%) from the superior mesenteric artery. The middle and right colic arteries supply the right half of the transverse colon and the ascending colon. Within the mesentery, the superior mesenteric artery courses to the right iliac fossa, curving to the left to end in the ileocolic artery by forming an anastomosis. Major side branches of the superior mesenteric artery originating usually on the right side are the inferior pancreaticoduodenal arteries supplying the lower part of the duodenum. These arteries connect with the superior pancreaticoduodenal arteries. The inferior pancreaticoduodenal artery can also arise from or can be in common with the first jejunal artery. To the left, four to six jejunal arteries and nine to thirteen intestinal branches that supply the ileum can be identified. These are often called “intestinal arteries.”
into two branches of unequal size at the level of the second or third sacral vertebra, commonly at the bottom of the pouch of Douglas. The larger right branch divides into several branches, which descend on the posterior and lateral surfaces of the rectal ampulla. The smaller left branch deviates to the left and supplies the lateral and anterior surfaces. In contrast to the small and large intestinal arteries, the branches of the rectal arteries do not form arcades but enter the gut wall directly and independently.
causes include adhesions or internal and external hernias causing strangulation and intussusception.
Table 2-1 Causes of Acute and Chronic Mesenteric Ischemia | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
uses oxygen and therefore, during ischemia, is unable to catalyze the conversion of hypoxanthine to xanthine, resulting in a buildup of excess tissue levels of hypoxanthine. When oxygen is reintroduced during reperfusion, conversion of the excess hypoxanthine by xanthine oxidase results in the formation of toxic reactive oxygen species (ROS). These are potent oxidizing and reducing agents that directly damage cellular membranes by lipid peroxidation. In addition, ROS stimulate leukocyte activation and chemotaxis by activating plasma membrane phospholipase A2 to form arachidonic acid. They also stimulate leukocyte adhesion molecule and cytokine gene expression via activation of transcription factors such as nuclear factor-κb. In addition to causing direct cell injury, ROS thus increase leukocyte activation, chemotaxis, and leukocyte-endothelial adherence. Ischemia reperfusion also results in complement activation and the formation of several proinflammatory mediators.
Table 2-2 Cellular Effects of Ischemia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
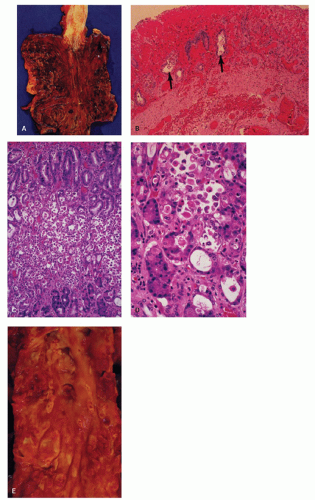
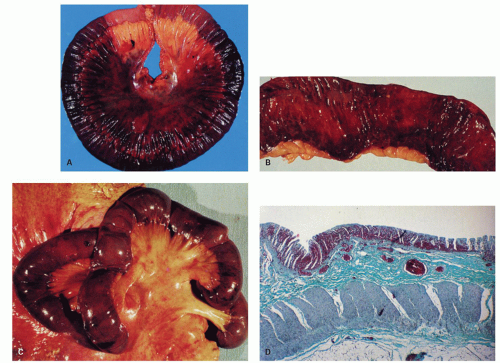
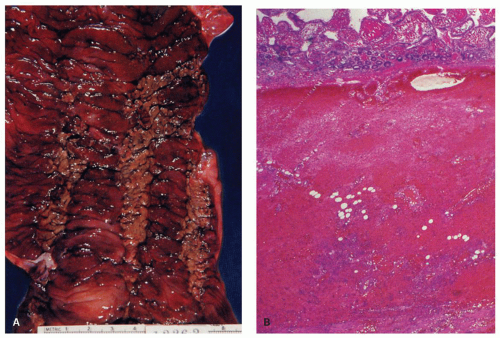
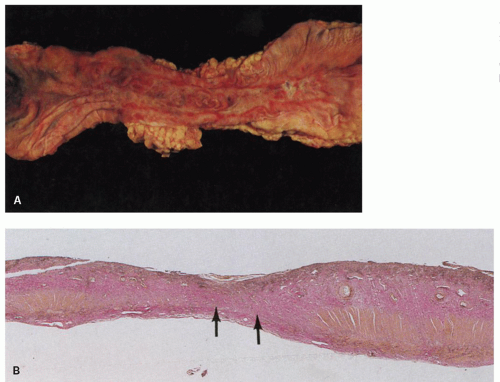
 Figure 2-4. Acute esophageal infarction. Gross appearance of the opened esophagus showing a diffusely dark and necrotic mucosa. |
and prompt therapeutic decisions. Intestinal ischemia accounts for an estimated 0.2% of all hospital admissions and 1% of all laparotomies. The true incidence of mesenteric ischemia is probably higher.37 The recent increase in acute mesenteric ischemia is most likely explained by improved abdominal imaging and by the aging population. Ischemia due to venous occlusion occurs in lower age groups. The symptoms and signs of acute mesenteric ischemia vary depending on the multitude of underlying vascular events. Arterial thromboembolism results in the most dramatic presentation, while the symptoms of NOMI and venous thrombosis tend to develop gradually over hours to days.38 The clinical picture of typical acute mesenteric ischemia can be subdivided into four stages.39 The hyperreactive state is characterized by severe abdominal pain in the absence of major abdominal findings at physical examination. In this stage there is hyperperistalsis and sudden vomiting or passage of loose stools. In the paralytic stage, the pain spreads over the abdomen and becomes continuous. The abdomen distends and loses its bowel sounds. The last two stages are nonspecific and are related to generalized peritonitis. In the third stage massive fluid and electrolyte losses occur due to capillary leak and a disturbed intestinal barrier. In the final stage shock rapidly evolves and the patient’s general state deteriorates rapidly. NOMI and venous thrombosis present more nonspecifically, and of all the early clinical signs only abdominal pain is seen in the majority of the patients. Recognition of vascular abdominal rest pain, defined as severe and persisting for more than 2 hours without recent caloric intake in the absence of peritoneal signs, is the key to early diagnosis.
Table 2-3 Major Causes of Acute Mesenteric Ischemia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
most of this relates to superior mesenteric artery territory, and involvement of inferior mesenteric territory is less dramatic, possibly due to collateral circulation. For the clinician, a key to early diagnosis of mesenteric ischemia or mesenteric venous thrombosis is to keep a low threshold to consider it in the differential diagnosis since symptoms or signs may be nonspecific. If clinicians wait for dramatic signs of mesenteric ischemia like lactic acidosis, it may be too late.
muscle coats are replaced by fibrous tissue, resulting in strictures, which may be concentric or eccentric, depending on the extent of the ischemic necrosis.
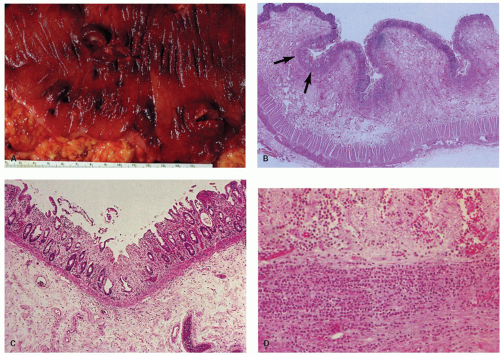
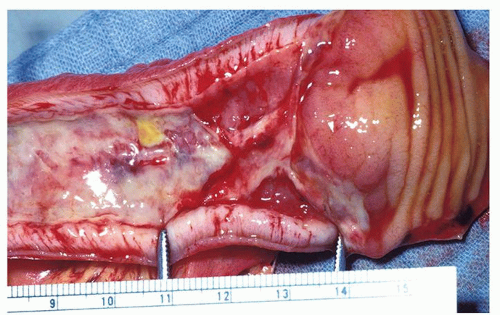 Figure 2-7. Segmental infarction of the terminal ileum showing a large mucosal ulceration. The demarcation between normal and involved segment is fairly sharp in arterial lesions as seen here. |
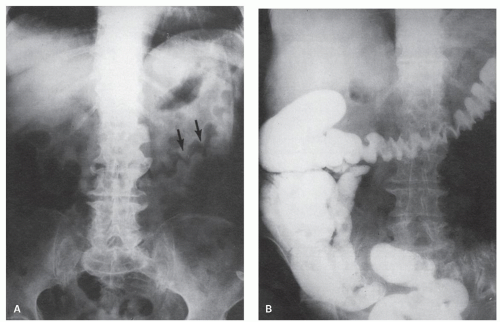 Figure 2-8. x-Ray appearances of ischemic colitis corresponding to the colon illustrated in Figure 2-9. A: Preliminary film showing the thickened mucosal folds, described as thumbprinting (arrows). B: Barium examination showing thickened mucosal folds. (Courtesy M. Weiner, M.D.) |
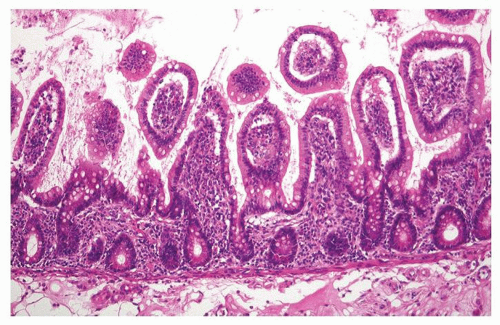
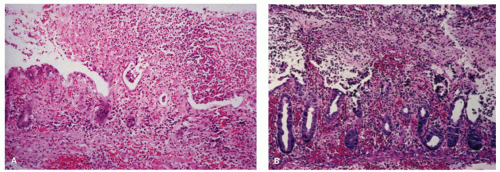
villi appear low cuboidal or flat with a basophilic cytoplasm. The crypts become mucin depleted, undergo coagulative necrosis, and eventually become detached from the basement membrane and extruded. Complete necrosis of the small intestinal mucosa in man is observed after about 44 hours of ischemia (Fig. 2-11).50
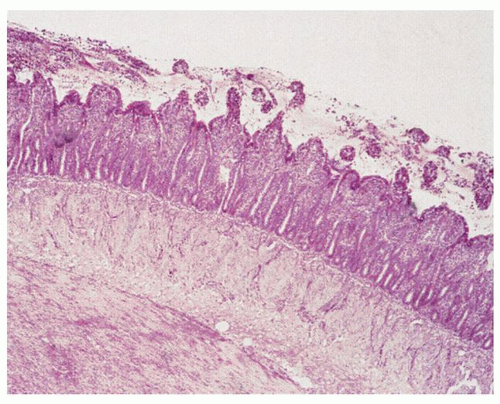
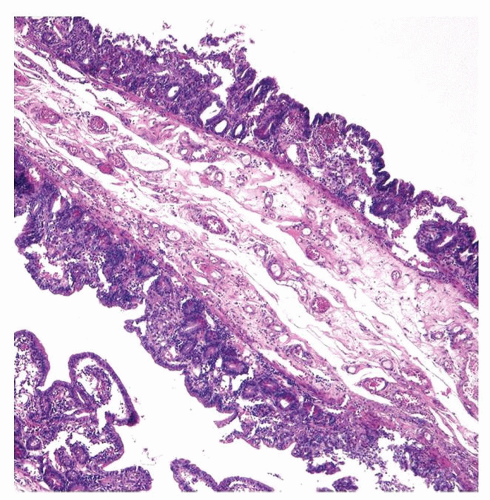 Figure 2-10. Diffuse flattening of the mucosa with loss of villi in the early recovery phase of small intestinal ischemic necrosis. |
Escherichia coli [E. Coli] O157:H7), or coagulopathies are at risk. Intestinal vasculitis rarely occurs in the absence of systemic manifestations of vasculitis.
Table 2-4 Etiology of Ischemic Colitis in Young Adults (n = 42) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
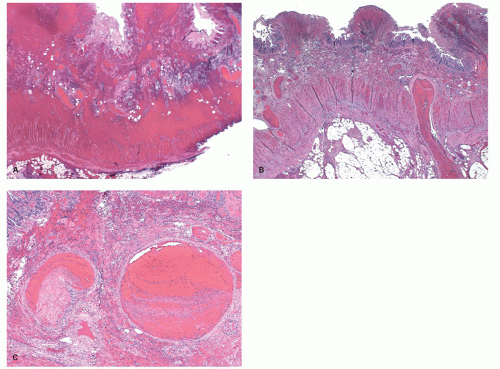
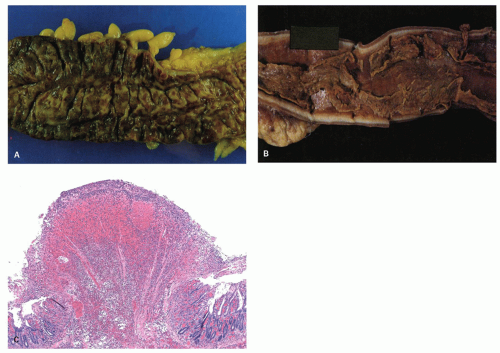
or, rarely, gangrene and perforation (Figs. 2-13 and 2-14). In some cases, ischemia produces pseudomembranes (Fig. 2-15) characterized by yellowish mucosal plaques. However, most of these cases occur in patients on antibiotic therapy and are thought to be caused by C. difficile colitis, but can be seen in other severe infections especially verotoxin-producing organisms.
crypts are dilated, and necrosis is mainly present in the upper half of the mucosa.86
by itself and represents about 10% of all cases of large bowel ischemia. Rectal ischemia occurs in patients with occlusive internal iliac artery disease. Acute presentation may follow the ligation of the inferior mesenteric artery (operations of the lower aorta) or other iatrogenic interventions including sclerotherapy for hemorrhoids.91 The endoscopic features and pathology of ischemic proctitis are the same as those of ischemic colitis.92, 93 It must be differentiated from other causes of rectal inflammation such as the solitary rectal ulcer syndrome or rectal lesions due to abuse of suppositories.
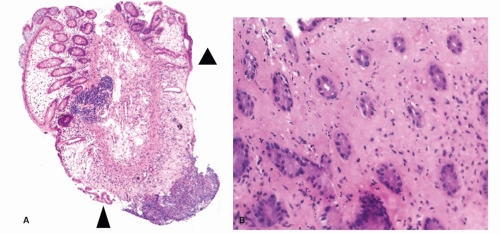
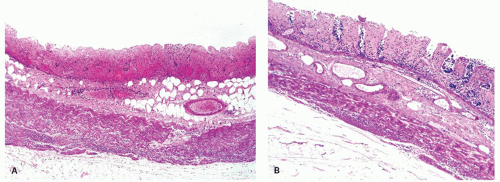 Figure 2-16. A: Extensive large bowel infarction with diffuse transmural necrosis and complete loss of glands. B: Epithelial cells can persist in some crypts. |
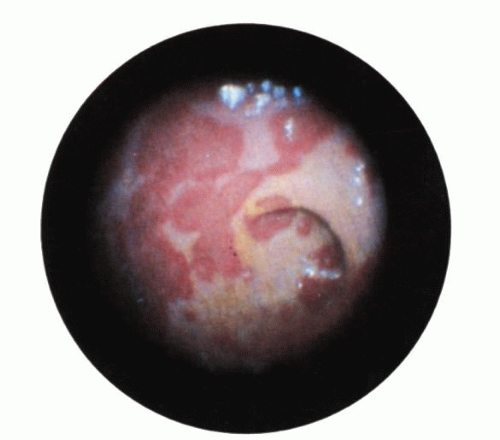 Figure 2-18. Ischemic colitis following aortofemoral bypass surgery. Endoscopic appearance showing a serpentine, white-based exudate in the rectum. |
biopsies of the GI tract are more likely to be negative as very little submucosa is included, and they merely show features of mucosal ischemia. Rectal biopsies were indeed negative in many patients with established systemic vasculitis.97 The establishment of the involvement of larger vessels requires surgical samples. The presence of isolated GI vasculitis is extremely uncommon in the absence of other systemic features of vasculitis.98
of the vessel wall, or both. However, certain changes such as extravasation of erythrocytes and edema due to leakiness, thrombosis, and infiltration of the vessel wall can occur without fibrinoid necrosis of the vessel wall.
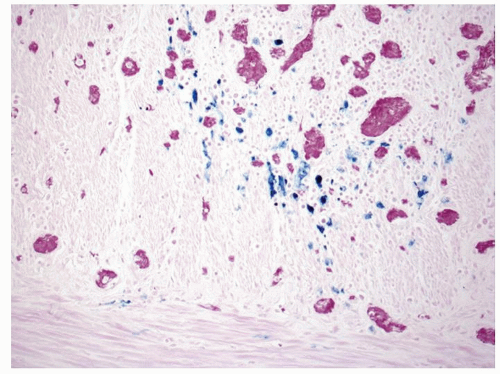 Figure 2-21. Recovery following ischemic damage can be characterized by abnormal crypt architecture and the presence of iron-laden, Perls-positive macrophages which are stained blue. |
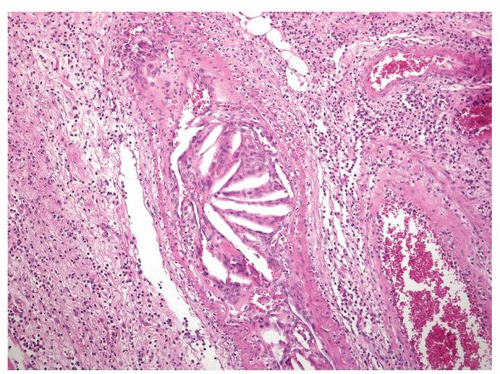 Figure 2-22. Cholesterol emboli that result from the release of material from atherosclerotic plaques in a submucosal artery. |
Table 2-5 Classification of Vasculitis According to Histologic Pattern | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
and the clinical presentation depends upon the size of the vessels involved as well as distribution of the disease. The protean clinical manifestations, combined with the etiologic nonspecificity of the histologic lesions, complicate the diagnosis of specific forms of vasculitis. This is problematic because different vasculitides with indistinguishable clinical presentation (e.g., Henoch-Schönlein and MPA) may have different prognosis and treatment. A general classification covering all these aspects and easily applicable in clinical practice is at present not available. One approach is to categorize the noninfectious vasculitides on the basis of the predominant type of vessel affected. Such a classification has been proposed by an international conference, and attempts have been made to combine the classification according to the vessel size and etiology (see Table 2-6).103 This was followed by a proposal for uniform terminology and definitions.104 It is clinically important to identify the type and size of inflamed vessels and to determine
whether the inflammation is focal or extensive.102, 104 These variables have an influence upon the clinical presentation and the diagnostic techniques used.94, 95 Involvement of larger vessels is frequently associated with more severe clinical syndromes; for example, involvement of larger “mesenteric vessels” results in complications such as bowel necrosis, infarction, and perforation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree