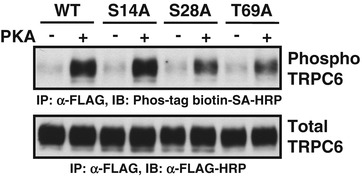
Fig. 1
Effects of phosphatase treatment on the basal phosphorylation of FLAG-tagged TRPC6 proteins immunoprecipitated with anti-FLAG antibody. Immunoprecipitated TRPC6 proteins were incubated in the dephosphorylation reaction mixture for 1 h. Upper panel is a representative immunoblot with Phos-tag biotin -bound HRP-SA complex detecting phosphorylated TRPC6 proteins (indicated as phospho TRPC6). Lower panel is a representative immunoblot with anti-FLAG-HRP antibody to determine the quantity of TRPC6 in the immunoprecipitate (indicated as total TRPC6). Reproduced with permission from Ref. [15]
19.
Place tube on magnet and remove supernatant.
20.
Wash the Dynabeads™-antibody-TRPC6-FLAG complex three times with 200 μL of the washing buffer by gently vortexing for each wash.
21.
Transfer the suspension to a clean tube.
22.
Place tube on magnet and remove supernatant.
23.
Add 25 μL of phosphorylation reaction mixture and incubate for 30 min at 37 °C with shaking at 200 rpm (see Fig. 2).
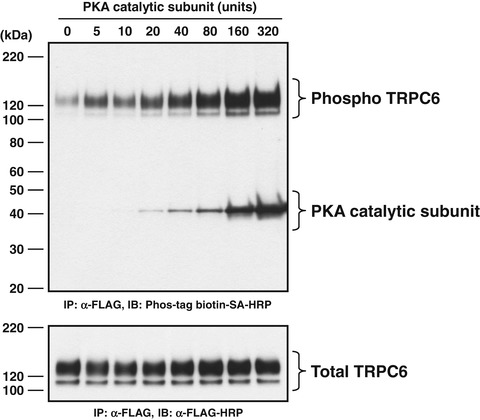

Fig. 2
Dose–response relationship for PKA-mediated phosphorylation of wild-type TRPC6 protein. Immunoprecipitated TRPC6 protein was incubated in the dephosphorylation reaction mixture for 1 h and then was phosphorylated with PKA catalytic subunit at indicated doses for 30 min. Upper panel is a representative immunoblot with Phos-tag biotin -bound HRP-SA complex to detect phosphorylated TRPC6 protein (indicated as phospho TRPC6). Lower panel is a representative immunoblot with anti-FLAG-HRP antibody to determine the quantity of TRPC6 in the immunoprecipitate (indicated as total TRPC6). The locations of the bands for TRPC6-FLAG and PKA catalytic subunit are indicated to the right. The positions of protein size markers are indicated to the left in kDa. Reproduced with permission from Ref. [15]
24.
Add 6 μL of SDS sample buffer (×5) and incubate for 30 min at 37 °C with shaking at 200 rpm (see Note 4 ).
25.
Place tube on magnet and transfer supernatant to a clean tube.
26.
Store at −80 °C before use.
3.3 Western Blot Analysis
1.
Carry out SDS-PAGE in 5–20 %, 13-well polyacrylamide gels. Apply 5 or 15 μL of immunoprecipitated sample to each well. Run the gel at 80 volts for 30 min, and then at 130 volts untill the BPB dye front has reached the bottom of the gel.
2.
Immediately following SDS-PAGE, transfer the proteins separated on the gel to a PVDF membrane at 100 volts for 60 min in a refrigerator kept at 4 °C.
3.
Wash the membrane three times with TBS-T, 5 min for each wash.
5.
Wash the membrane two times with TBS-T, 3 min for each wash.
6.
Incubate the membrane with Zn2+-Phos-tag™ biotin -bound HRP-SA solution at room temperature for 6 h (for 15 μL of immunoprecipitated sample per lane) or with anti-FLAG-HRP antibody (1:6000 dilution, diluted in blocking buffer, anti-DDDDK-tag HRP-DirecT) overnight at 4 °C (for 5 μL of immunoprecipitated sample per lane).
7.
Wash the membrane three times with TBS-T, 5 min for each wash.
8.
Detect Zn2+-Phos-tag™ biotin -bound HRP-SA and anti-FLAG-HRP antibody with ECL Western blotting analysis system and ECL film (see Figs. 1, 2, and 3).