Using Magnetic Resonance Contrast Agents in the Abdomen and Pelvis
Intravenous Contrast Agents
Intravenous magnetic resonance (MR) contrast agents are essential for the majority of abdominal and pelvic indications. Three main types of intravenous contrast agents are currently available for widespread clinical use: gadolinium chelates, manganese chelates, and superparamagnetic iron oxide. New agents are constantly being developed and tested, and new indications are being approved for existing agents. Therefore, one should always seek the most current information on approval status, indications, contraindications, and approved dosing. One should also be familiar with the package insert before administering any intravenous contrast agent.
Gadolinium Chelates
Four extracellular gadolinium-based agents have been approved for use in the United States. These agents are gadopentetate dimeglumine (Magnevist), gadodiamide (Omniscan), gadoteridol (ProHance), and gadoversetamide (OptiMARK). Of these agents, gadopentetate dimeglumine is ionic and the others are nonionic. Aside from this difference, the gadolinium chelates are very similar in most respects. After intravenous administration, these agents all equilibrate rapidly with the extracellular fluid space. They are all hyperosmolar to plasma, and none bind to serum proteins. All of the agents are eliminated almost entirely via renal excretion and are dialyzable. No clinically significant biliary excretion occurs with any of the gadolinium chelates listed. There are no significant differences in the imaging characteristics of these agents.
Other gadolinium chelates, none of which are currently approved for use in the United States, exhibit some hepatobiliary activity. These agents have the theoretical advantage for liver imaging of combining the benefits of an extracellular agent (e.g., vascular phase imaging) with the advantages of a hepatocyte-specific agent. One such agent, gadobenate dimeglumine (MultiHance), is currently available in Europe, but demonstrates only modest hepatic parenchymal retention and biliary excretion (5%). Another hepatobiliary agent under clinical development is gadolinium EOB-DTPA (Eovist). This agent has an ethoxybenzyl group that results in greater hepatocyte uptake and biliary excretion (roughly similar to its renal excretion) than achieved by gadobenate dimeglumine.
Mechanism of Action
Gadolinium chelates currently approved for use in the United States are paramagnetic extracellular agents. The paramagnetic nature of gadolinium causes shortening of the T1 relaxation time of nearby water protons, which results in increased signal intensity of tissues on T1-weighted images. In other words, the effect of gadolinium chelates on tissue is indirect. Because gadolinium chelates are extracellular agents, they do not cross cell membranes. Instead, these agents become distributed throughout the intravascular and interstitial spaces. In this respect, the extracellular gadolinium chelates behave in a similar manner to the iodinated contrast agents used for computed tomography (CT).
Imaging Appearance
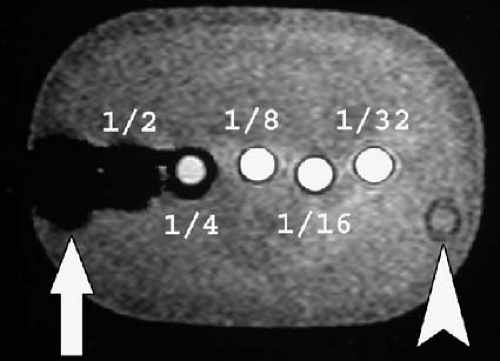
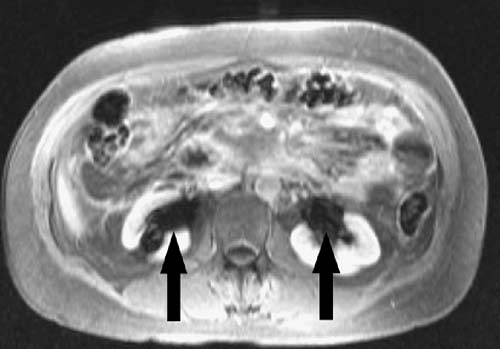
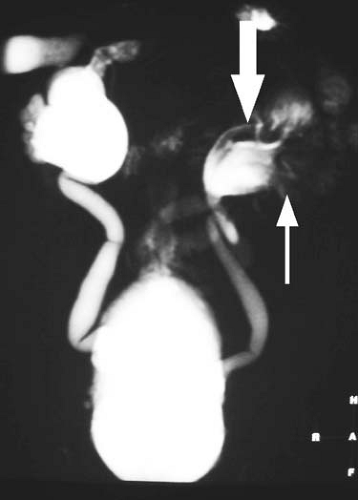
Paramagnetic agents shorten the T1 relaxation time of nearby tissues, causing these tissues to appear brighter on T1-weighted MR images. Although paramagnetic agents also shorten T2 relaxation times, the T1 shortening effect predominates at typical tissue concentrations found after intravenous injection of a routine clinical dose. At higher concentrations, the T2 shortening effect predominates, resulting in signal loss (Figs. 1.31, 1.32). This effect is commonly seen in the renal collecting systems and bladder, where the gadolinium concentration increases markedly after contrast administration due to renal excretion of the contrast agent.
Indications
The indications for gadolinium-enhanced MR imaging (MRI) are similar to those for contrast-enhanced CT. In broad terms, the indications for intravenous contrast material include the detection and characterization of abdominal and pelvic pathology and the depiction of the abdominal and pelvic vasculature. Both sensitivity and specificity are improved for detection of most disease processes with the use of gadolinium chelates. We routinely use gadolinium for most hepatic, renal, pancreatic, and vascular examinations. We selectively use gadolinium for adrenal and pelvic imaging. Not all gadolinium chelates are currently approved for all indications and age groups; refer to the appropriate package insert for the list of approved indications.
Contraindications and Special Clinical Scenarios
It is controversial whether cross-reactivity between gadolinium agents and iodinated contrast material exists. Therefore, caution should be exercised when administering gadolinium chelates to patients with a history of allergy or hypersensitivity to iodinated contrast. The use of prophylactic steroid treatment in patients allergic to iodinated contrast or gadolinium chelates before the administration of gadolinium chelates has not been thoroughly evaluated.
Significant nephrotoxicity has not been reported with the gadolinium chelates. Because these agents are dialyzable, they can be used safely in patients on renal dialysis. Multiple regularly scheduled dialysis sessions are required to clear the agent completely. The acceptable maximum duration between gadolinium administration and a patient’s first dialysis session has not been established. Gadolinium chelates may be safely used in patients with hepatic dysfunction.
Gadolinium chelates are category C agents in the setting of pregnancy and should only be administered to pregnant women when the potential benefits clearly justify the potential risk to the fetus. Some preparations of gadolinium have been shown to cross the placenta and small amounts may be excreted in breast milk (1). However, gadolinium is poorly absorbed via the gastrointestinal tract, and there have been no reported adverse effects on an infant related to breast-feeding after maternal administration of an approved dose of gadolinium chelate. Despite the sparse data supporting this practice, many MR practitioners believe it may be prudent to temporarily interrupt breast-feeding for at least 24 hours following the administration of gadolinium to lactating women until more definitive safety data are available.
Intravenous gadolinium chelates pose a theoretical risk in patients with sickle cell anemia. Deoxygenated sickle erythrocytes align perpendicularly to an external magnetic field in vitro. It is feasible that paramagnetic contrast agents may potentiate this effect in vivo, possibly leading to vasoocclusive complications. Once again, no strong in vivo evidence exists to validate this concern.
Dose and Administration
The standard adult intravenous dose of currently available gadolinium chelates for abdominal and pelvic applications is 0.1 mmol/kg body weight administered at a rate of 1 to
2 mL/sec. A dose of 0.3 mmol/kg body weight should not be exceeded. The standard 0.1 mmol/kg dose also applies to pediatric patients for those agents approved for pediatric use. Not all intravenous gadolinium preparations have been approved for doses greater than 0.1 mmol/kg, rapid bolus injection, or multiple doses. Once again, we recommend checking the relevant package insert for more information before administering these agents.
2 mL/sec. A dose of 0.3 mmol/kg body weight should not be exceeded. The standard 0.1 mmol/kg dose also applies to pediatric patients for those agents approved for pediatric use. Not all intravenous gadolinium preparations have been approved for doses greater than 0.1 mmol/kg, rapid bolus injection, or multiple doses. Once again, we recommend checking the relevant package insert for more information before administering these agents.
Pitfalls
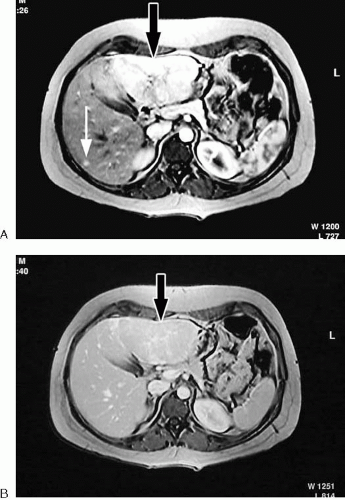
The goal of administering intravenous contrast agents for abdominal and pelvic MR applications is to increase the diagnostic efficacy of the examination. Contrast agents typically accomplish this goal by increasing lesion conspicuity. In many cases, the target lesion is a tumor within a solid organ, and tumors vary in enhancement characteristics based on their histologic properties and blood supply. Some tumors are most conspicuous during the arterial or venous phase of imaging, whereas other tumors are more conspicuous on precontrast images, becoming isointense to surrounding parenchyma after contrast administration. Therefore, an appropriately timed multiphase examination that includes precontrast images is critical to lesion detection in abdominal organs such as the liver and pancreas (Fig 1.33).
In most cases, fat suppression is recommended for postcontrast imaging with gadolinium. Fat suppression aids in improving image contrast between enhancing structures and the surrounding fat, particularly at the edges of organs. Keep in mind that non-radiofrequency (RF)-selective inversion recovery fat suppression techniques such as short tau inversion recovery (STIR) are not suitable for post-gadolinium imaging, because enhancing tissues can be suppressed along with other tissues having a short T1.
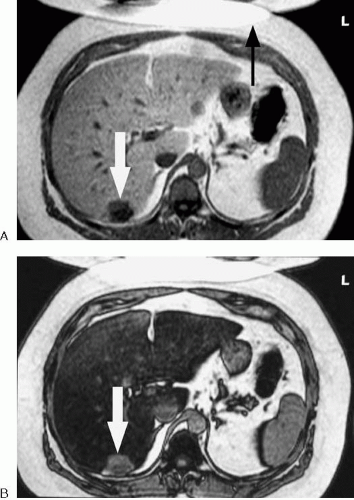
Some dynamic MR sequences are performed with an opposed-phase echo time (TE). In the setting of fatty infiltration of the liver, a nonenhancing lesion that is dark on precontrast in-phase T1-weighted images may exhibit pseudoenhancement when an opposed-phase dynamic contrast-enhanced scan is performed. The apparent enhancement of the lesion results from signal loss in the surrounding liver and not from contrast uptake by the cyst. Comparison to the precontrast images or subtracting the precontrast from postcontrast images usually resolves this issue (Fig 1.34).
Blood products may appear bright on a T1-weighted image due to the T1 shortening effects of methemoglobin. Therefore, a hematoma may be difficult to distinguish from an enhancing tumor if precontrast T1-weighted images are not obtained.
The T2 shortening effect of gadolinium may result in signal loss once gadolinium becomes sufficiently concentrated in a tissue. Therefore, for indications such as MR urography, it is important to reduce the dose of contrast administered, image quickly before the contrast becomes too concentrated, or hydrate the patient well to ensure dilution of the contrast within the renal collecting systems and ureters (Figs. 1.32, 1.35). Similarly, gadolinium administered for direct MR venography must be diluted before administration.
Dynamic Imaging with Gadolinium Chelates
Indications
Rapid scanning techniques have ushered in the era of dynamic MRI of the abdomen and pelvis. As with CT, one can repeatedly image entire organs, achieving discrete temporal separation of vascular phases. In addition to increasing sensitivity for lesion detection, dynamic imaging improves the specificity of lesion characterization based on the pattern of contrast enhancement (4). In general, dynamic contrast-enhanced imaging is routinely recommended for the liver, pancreas, and kidneys. Evaluation and staging of neoplasms is often enhanced through the use of dynamic imaging. In addition, dynamic imaging provides “free” information about
the vascular anatomy. Because MRI does not involve the use of ionizing radiation, there is no increased risk associated with dynamic imaging other than the very small risk of administering an intravenous gadolinium chelate. Currently, dynamic contrast-enhanced MRI is only performed with gadolinium chelates, which can be injected rapidly. The currently approved manganese and iron-based contrast agents require a slower rate of administration.
the vascular anatomy. Because MRI does not involve the use of ionizing radiation, there is no increased risk associated with dynamic imaging other than the very small risk of administering an intravenous gadolinium chelate. Currently, dynamic contrast-enhanced MRI is only performed with gadolinium chelates, which can be injected rapidly. The currently approved manganese and iron-based contrast agents require a slower rate of administration.
Technique
For dynamic imaging to be performed, the gadolinium chelate must be injected rapidly. Favored injection rates vary, although a rate of 2 mL/sec works well for most applications. A mechanical injector is not critical for dynamic imaging, but it is convenient and ensures uniformity and consistency. An experienced technologist can hand-inject contrast material and approximate the desired rate, although this requires the presence of a second person to initiate the scan at the appropriate time. Typically, injection of the contrast medium is followed by a saline flush of 15 to 20 mL. Successful dynamic imaging depends on adequate timing of the acquisition relative to the arrival of the contrast bolus. Typically, four sets of images are acquired: precontrast, arterial phase, portal venous phase, and equilibrium phase (Fig. 1.36). To achieve adequate temporal resolution and cover the entire organ of interest in a single breath-hold, the acquisition time for each phase should be kept as short as possible (while maintaining adequate spatial resolution and signal-to-noise ratio). On newer scanners, this can be easily achieved with acquisition times of less than 30 seconds per phase. Dynamic imaging can be performed with either a two-dimensional (2D) or a three-dimensional (3D) acquisition.
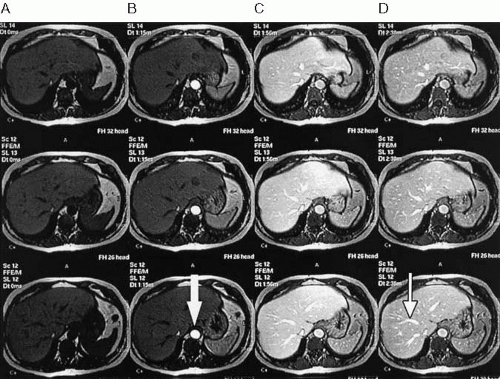 FIG. 1.36. Dynamic gadolinium-enhanced imaging. One precontrast (A) and three postcontrast (B-D) scans of upper abdomen were performed following bolus administration of intravenous gadolinium. Each T1-weighted gradient echo scan covered entire liver during breath-hold. Note varying signal intensity of hepatic parenchyma, aorta (arrow), and venous structures (thin arrow).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|