Fig. 1
Recommended chemistry setup for filling apoferritin . (a) Double manifold importing N2 gas and expelling nitrogen through a gas bubbler (b). (c) Three neck flask fitted with a coil condenser to prevent solvent loss and an infusion needle to inject the iron, sealed with sleeve septa. (d) FeCl2 solution degassing separately. (e) Water bath at 60–65 °C warming the three neck flask and apoferritin solution. (f) Syringe pump to inject the iron solution at 16 μL/min. Arrows show the direction of N2 flow
1.
Prepare a round-bottom flask containing 20 nmol (9.15 mg) apoferritin in 10 mL 0.05 M MES, adjusted to pH 8.5.
2.
Prepare a second round-bottom flask containing excess of 50 mM Fe(II)Cl2.
3.
Place both flasks into a water bath or heat blanket to maintain a temperature of 60–65 °C.
4.
Degas both flasks by attaching a sleeve septum to the flask opening and sealing around the edges with paraffin film. Insert a needle attached to a tube leading to a regulated nitrogen tank into one septum and another through a nitrogen bubbler. Add nitrogen to the container at 50 psi. Slowly reduce the pressure of the gas until no bubbles are formed in the liquid of the flask.
5.
Carefully draw excess de-aerated FeCl2 solution into a 5 mL syringe fitted with an infusion needle, and then quickly transfer the needle to the septum on the center neck of the flask. Using a syringe pump, inject the FeCl2 into the apoferritin at a rate of 16 μL/min for 75 min (3000 Fe/ferritin ). You may adjust this time between 25 and 125 min (1000–5000 Fe/ferritin); increasing the time typically increases transverse MR relaxivity (r2), but decreases yield.
6.
Dialyze the ferritin -containing solution overnight (12 h) against 0.15 M NaCl using dialysis tubing (8000 Da MWCO).
7.
Measure final protein concentration using the Better Bradford Assay™ by UV/visible spectrophotometer absorbance at 595 nm.
8.
Measure MR relaxivity using a Bruker minispec™ or equivalent, at the desired field strength and MR acquisition parameters. Relaxivity can be measured in solution or in low melting point agar, with the temperature kept below 50 °C to avoid denaturing the ferritin protein.
9.
Store at 4 °C.
3.2 Cationized Ferritin Synthesis
We synthesize CF in our lab using a protocol adapted from Danon et al. [11]. All procedures should be performed in a chemical safety cabinet, following all safety protocols for the chemicals. This synthesis is for 27.5 mg, approximately 4 mg/mL. Volumes can be scaled as needed (see Note 5 ).
1.
Add 1.5 mL of deionized water in a 20 mL beaker.
2.
Slowly add 0.5 mL of stock 8 M (~99 %, ~200 DMPA/COO-) N,N-dimethyl-1,3,propanediamine (DMPA) to the beaker under stirring.
3.
Add 4 mL 2N HCl, then adjust to pH 6.0 with 0.2 N HCl and 0.2 N NaOH.
4.
Add 0.5 mL 55 mg/mL native ferritin to the solution of DMPA.
5.
Add 200 mg 1-ethyl-3(3-dimethyl-aminopropyl) carbodiimide hydrochloride (EDC, ~50 DMPA/COO-).
6.
Carefully maintain pH at 6 for 1 h. The addition of EDC will make the solution slightly basic, approaching 6.5, so it can be brought back down by addition of 0.2 N HCl. Take care to avoid adding too much HCl, as a pH below 4.0 can denature ferritin .
7.
Once pH is stabilized, cover with paraffin film and stir at room temperature overnight (12 h).
8.
Dialyze at least two times in 8000 Da MWCO dialysis bags against 3.5 L of PBS at 4 °C for 12–24 h each time.
9.
Store CF at 4 °C. The solution can be filtered through a syringe filter to remove aggregates before use.
10.
Measure concentration using a Better Bradford™ assay or equivalent.
3.3 Validating Cationization
To test the effectiveness of the cationization reaction, determine the number of carboxylate residues before and after the reaction by titration [11]. In our experience the titrated sample can be brought back to pH 7.2 with 0.1 N HCl, dialyzed into PBS, and used as normal.
![$$ \left[{\mathrm{COO}}^{-}\right]=\frac{\left({V}_{\mathrm{fn}}-{V}_{\mathrm{b}}\right)\times \left[\mathrm{H}\mathrm{C}\mathrm{l}\right]}{M_{\mathrm{fn}}}. $$](/wp-content/uploads/2016/11/A321592_2_En_7_Chapter_Equ1.gif)
Variables: [COO−] = titratable carboxylates (μmol/mg ferritin ), M fn is the mass of ferritin being titrated (mg), V fn and V b = volume of HCl solution required to decrease the pH from 5.2 to 3.0 (mL), [HCl] = concentration of the HCl solution (10 μmol/mL).
1.
Add 1–10 mg of ferritin or cationized ferritin to 5 ml diH2O (using more ferritin will give a more accurate result). For the buffer standard, add the same volume of buffer (typically saline or PBS) to 5 ml diH2O (see Note 6 ).
2.
Make a stock solution of aqueous 10 mM HCl.
3.
Slowly decrease the pH of the ferritin or buffer solution to 5.2 with HCl.
4.
Continue adding HCl solution in 1 μL increments, measuring the total volume (V ferritin or V buffer) of HCl solution required to decrease the pH from 5.2 to 3.0.
5.
To calculate the number of titratable carboxylate groups per mg of ferritin molecule:
![$$ \left[{\mathrm{COO}}^{-}\right]=\frac{\left({V}_{\mathrm{fn}}-{V}_{\mathrm{b}}\right)\times \left[\mathrm{H}\mathrm{C}\mathrm{l}\right]}{M_{\mathrm{fn}}}. $$](/wp-content/uploads/2016/11/A321592_2_En_7_Chapter_Equ1.gif)
(1)
The average surface charge per ferritin , 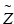 , can be estimated from the known amino acid sequence of horse spleen ferritin [NCBI, PDB: 1IER_A] (MW = 476.4 kDa, Asp 288, Glu 360, Lys 216, Arg 264).
, can be estimated from the known amino acid sequence of horse spleen ferritin [NCBI, PDB: 1IER_A] (MW = 476.4 kDa, Asp 288, Glu 360, Lys 216, Arg 264).
![$$ \mathrm{S}\mathrm{F}=\left|\frac{{\left[{\mathrm{C}\mathrm{OO}}^{-}\right]}_{\mathrm{M},\mathrm{N}\mathrm{F}}\times {\mathrm{M}\mathrm{W}}_{\mathrm{NF}}}{A_{\mathrm{C},\mathrm{N}\mathrm{F}}}\right|, $$](/wp-content/uploads/2016/11/A321592_2_En_7_Chapter_Equ2.gif)
where
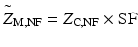
and
![$$ {\overset{\sim }{Z}}_{\mathrm{CF}}={\overset{\sim }{Z}}_{\mathrm{NF}}+2\times \left({\left[{\mathrm{COO}}^{-}\right]}_{\mathrm{M},\mathrm{N}\mathrm{F}}-{\left[{\mathrm{COO}}^{-}\right]}_{\mathrm{M},\mathrm{C}\mathrm{F}}\right)\times {\mathrm{M}\mathrm{W}}_{\mathrm{NF}} $$](/wp-content/uploads/2016/11/A321592_2_En_7_Chapter_Equ4.gif)
Variables: SF = surface fraction (unitless), [COO−] = carboxylate/mg (μmol/mg), MW = molecular weight (476.4 mg/μmol), A = calculated carboxylates per ferritin (−648 q), 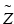 = average surface charge per ferritin (q), Z = calculated total charge per ferritin (−168 q) (see Note 7 ). Subscripts: M = measured, C = Concentration.
= average surface charge per ferritin (q), Z = calculated total charge per ferritin (−168 q) (see Note 7 ). Subscripts: M = measured, C = Concentration.
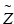 , can be estimated from the known amino acid sequence of horse spleen ferritin [NCBI, PDB: 1IER_A] (MW = 476.4 kDa, Asp 288, Glu 360, Lys 216, Arg 264).
, can be estimated from the known amino acid sequence of horse spleen ferritin [NCBI, PDB: 1IER_A] (MW = 476.4 kDa, Asp 288, Glu 360, Lys 216, Arg 264).![$$ \mathrm{S}\mathrm{F}=\left|\frac{{\left[{\mathrm{C}\mathrm{OO}}^{-}\right]}_{\mathrm{M},\mathrm{N}\mathrm{F}}\times {\mathrm{M}\mathrm{W}}_{\mathrm{NF}}}{A_{\mathrm{C},\mathrm{N}\mathrm{F}}}\right|, $$](/wp-content/uploads/2016/11/A321592_2_En_7_Chapter_Equ2.gif)
(2)
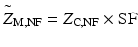
(3)
![$$ {\overset{\sim }{Z}}_{\mathrm{CF}}={\overset{\sim }{Z}}_{\mathrm{NF}}+2\times \left({\left[{\mathrm{COO}}^{-}\right]}_{\mathrm{M},\mathrm{N}\mathrm{F}}-{\left[{\mathrm{COO}}^{-}\right]}_{\mathrm{M},\mathrm{C}\mathrm{F}}\right)\times {\mathrm{M}\mathrm{W}}_{\mathrm{NF}} $$](/wp-content/uploads/2016/11/A321592_2_En_7_Chapter_Equ4.gif)
(4)
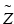 = average surface charge per ferritin (q), Z = calculated total charge per ferritin (−168 q) (see Note 7 ). Subscripts: M = measured, C = Concentration.
= average surface charge per ferritin (q), Z = calculated total charge per ferritin (−168 q) (see Note 7 ). Subscripts: M = measured, C = Concentration.3.4 Cationized Ferritin Labeling and MRI in Rat Kidneys
For all experiments, obtain regulatory approval from the relevant institutional animal care and use committee.
1.
Weigh and prepare rats for intravenous injections.
2.
Inject CF intravenously (e.g., by tail vein or penile vein). Typical bolus injections are performed with a total of 5.75 mg of CF per 100 g per body weight, with injected concentrations of 11 mg/mL. It is common to deliver the boluses in three equal injections of 1 ml spaced 1.5 h apart (2 × the blood half-life of CF). Toxicity manifests as systemic hypoxia , characterized by a blue skin tone in the feet and tail.
3.
1.5 h after the last injection, anesthetize the rat with either isoflurane or an equivalent (e.g., ketamine/xylazine cocktail), matched to the weight of the animal.
4.




Incise the chest and abdomen of the rat to expose the heart, liver, and kidneys. Excise the right lung or make an incision in the right ventricle of the heart. Insert a large-gauge needle (18–21 gauge) into the left ventricle of the heart and systemically perfuse the rat using a peristaltic pump to drive the perfusate through the needle and into the vasculature. Perfuse first with saline at a rate of at least 100 mL/min (see Note 8 ) for a total volume of 400–500 mL. Confirm no visible blood in the kidneys, then perfuse with 10 % neutral buffered formalin at a rate of ~100 mL/min for a total of 100 mL of formalin (see Note 9 ). Remove the kidneys and place into fixative (see Note 3 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







