Age at diagnosis
66–74 Years
75+ Years
5-Year mortality
10-Year mortality
5-Year mortality
10-Year mortality
Characteristic
Rate per 100a
95% CIb
Rate per 100a
95% CIb
Rate per 100a
95% CIb
Rate per 100a
95% CIb
T1c, Gleason 5–7, co morbidity = 0
Overall mortality
11.7
10.2–13.1
28.8
25.3–32.6
26.3
24.8–28.0
67.1
63–72.4
Prostate cancer specific mortality
1.6
1.1–2.4
4.8
2.8–8.4
4.4
3.4–5.1
14.0
10.6–20.9
T1c, Gleason 5–7, co morbidity = 1
Overall mortality
25.3
20.7–29.5
50.5
41.5–59.2
39.4
35.9–42.5
76.8
70.5–82.9
Prostate cancer-specific mortality
1.1
0.0–2.7
2.0
0.0–5.3
5.1
3.3–7.2
9.1
5.5–14.4
T1c, Gleason 5–7, co morbidity ≥2
Overall mortality
42.5
36.1–48.5
83.1
67.4–97.2
48.1
42.7–52.7
74.4
63.7–84.7
Prostate cancer-specific mortality
4.3
1.6–8.3
5.3
2.5–10.0
4.0
1.7–6.5
5.0
2.5–8.7
T1c, Gleason 8–10, co morbidity = 0
Overall mortality
26.4
22.2–30.8
55.0
43.9–65.9
41.4
38.3–44.0
77.0
71.5 to 82.5
Prostate cancer specific mortality
13.6
9.6–17.8
25.7
15.9–40.6
16.3
13.8–19.4
27.5
21.5 to 36.5
T1c, Gleason 8-10, co morbidity = 1
Overall mortality
30.7
23.7–41.0
52.0
38.0–77.0
47.2
41.8–52.5
92.4
79.3 to 99.7
Prostate cancer-specific mortality
11.6
3.0–23.4
20.2
4.1–46.6
11.2
7.3–16.1
23.7
9.5 to 44.7
T1c, Gleason 8–10, co morbidity ≥2
Overall mortality
52.0
42.1–64.5
64.3
52.0–84.9
65.7
55.9–70.1
94.3
87.4–100.0
Prostate cancer-specific mortality
9.6
2.4–19.3
13.7
2.7–33.4
12.8
7.3–18.9
18.8
9.3–36.8
PSA Screening in the Elderly
Since the introduction and widespread implementation of PSA screening in the early 1990s there has been a significant prostate cancer stage migration, favoring the diagnosis of low-grade, low-stage disease [21]. This stage migration, coupled with the remarkable prevalence of histologic prostate cancer at autopsy, has prompted many clinicians and patients alike to question the necessity of PSA screening. Two recent randomized trials reported interim survival results detailing the effectiveness of PSA screening on both overall and prostate cancer-specific mortality. The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) randomized 76,693 men to yearly prostate cancer screening with PSA and digital rectal examination (DRE) for 6 and 4 years, respectively. The risk of prostate cancer diagnosis was 116 per 10,000 person-years and 95 per 10,000 person-years in the screening and control groups, respectively. The risk of prostate cancer death was found to be 2.0 per 10,000 person-years and 1.7 per 10,000 person-years in the screening and control groups, respectively indicating both a very low rate of prostate cancer death and a lack of protection conferred by prostate cancer screening [22]. The PCLO study has been widely criticized due to the high rate of contamination found of the control group. Specifically, 52% of the patients randomized to the control arm of the study were screened with PSA testing by year 6, leading many to surmise that, while the survival benefit of PSA screening may indeed be small, this effect is largely attenuated by the high rate of screening in the control arm of the PLCO trial. The European Randomised Study of Screening for Prostate Cancer (ERSPC) randomized 182,000 men between the ages of 50 and 74 years to prostate cancer screening or no prostate cancer screening. Unlike the PLCO study, screening in the European randomized trial occurred, on average, every 4 years. The risk of prostate cancer diagnosis was found to be 8.2% and 4.8% in the screening and control arms, respectively. In the overall cohort, prostate cancer screening conferred a 20% reduction in the risk of prostate cancer-mortality. Excluding noncompliant study participants, the prostate cancer-specific mortality benefit improved to 27%. Despite these findings, investigators determined that 1,410 men would have to be screened and 48 men treated to prevent one prostate cancer death [23]. Despite the need to screen and treat a large number of men to prevent few prostate cancer deaths, many believe that longer follow-up will result in a larger observed survival benefit with PSA screening. Modeling the number needed to screen (NNS) and number needed to treat (NNT) in the ERSPC study reveals that, by 12 years of follow-up, the NNS and NNT will be 503 and 18, respectively [24].
What are the explanations for the disparate findings in the PLCO and ERSPC studies? Most point to two significant differences between the studies, namely vastly different rates of PSA screening/contamination of the control groups, and the variable rates of PSA “prescreening” amongst study participants. Indeed, nearly half of the patients assigned to the control arm of the PLCO study were subject to PSA screening during the trial likely leading to the modest increase in prostate cancer detection (17%) in the screening arm compared to the control arm. The rate of contamination of the ERSPC study was significantly less, and the incremental increase in prostate cancer detection in the screening arm was much higher than the PCLO study (71%). Additionally, half of the patients in the PLCO study underwent PSA testing and digital rectal exam (DRE) within 3 years of enrollment leading many to surmise that the prescreened nature of the control arm of the PCLO study attenuated any meaningful difference in prostate cancer-specific mortality [25].
Given the challenges in the interpretation of the recent randomized trials, not surprisingly, the appropriate use of PSA screening in older age males remains even less clear. Indeed, the risk of prostate cancer overdetection increases with increasing age with PSA testing resulting in an overdetection rate of 27% at 55 years and 56% by 75 years [17]. Nonetheless, the likelihood of high-risk prostate cancer increases with age accounting for 43% of tumors diagnosed in men older than 75 years as compared to 25% of tumors diagnosed in men younger than 75 years [26]. Assessment of individual comorbidity, family longevity, and overall health status in addition to ascertainment of patient-specific attitudes towards screening and treatment is necessary in order to allow patients to make well-informed decisions surrounding participation in PSA screening. While no consensus guidelines exist to direct physicians as to when to discontinue prostate cancer screening in older men, the U.S Preventative Services Task Force (USPSTF) has recommended discontinuing PSA screening in those patients older than 75 years of age [27–29]. Critics of the USPSTF guidelines argue that patients should be assessed for overall health based upon individual factors and not upon chronological age. Indeed, 78% of men surveyed at a prostate cancer screening clinic disagreed with the recommendation to discontinue screening at age 75 [30]. Given the limitations of the USPSTF recommendations, the most recent American Urological Association best practice statement advocates for an individualized approach to prostate cancer screening in patients of advanced age given the heterogeneity of overall health status amongst elderly patients [28]. Despite little evidence to support the benefit of PSA screening in elderly populations, the prevalence of screening in the USA population remains high [31]. In fact, some series indicate that the rate of screening is higher in those patients over age 70 than in those patients in their 50s [32]. Nonetheless, some evidence indicates that the rate of PSA testing among men older than 75 years declined after the publication of the U.S. Preventative Services Task Force recommendation and has continued to decline since the publication of the PLCO and ERSPC studies [33].
Considering the challenges imposed by PSA screening in the elderly, substantial effort has been directed at identifying very low-risk populations of elderly patients in whom PSA screening can be withheld. A recent longitudinal cohort study of men undergoing PSA screening revealed that no men between 75 and 80 years of age with a PSA of <3.0 ng/mL died of prostate cancer, potentially identifying a subset of men in which PSA screening could be safely omitted [34]. Additional similar identifying variables would benefit this and younger age groups.
Treatment of Localized Prostate Cancer
Most prostate cancers currently detected in the U.S. are clinically localized. Although many such men have low- or intermediate-risk disease, a significant majority seek active treatment [21]. Indeed, recent data suggests that 81.7% of men older than 75 receive active treatment for newly diagnosed prostate cancer. While a higher proportion of patients with high-risk disease receive active treatment (86.4%) when compared to those with low-risk disease (72.2%), this difference is modest [35]. The decision surrounding whether to undertake active treatment for localized prostate cancer in an older man must take into account the likelihood of death from prostate cancer, the risk of symptomatic metastatic disease, and the risk of death from other causes. Observational data suggests that elderly men do enjoy more favorable overall survival when treated for low- and intermediate-risk prostate cancer. Indeed, men 65–80 years old who underwent active treatment for prostate cancer were found to have a statistically significant survival advantage when compared to men that did not undergo active treatment (HR 0.69, 95% CI 0.66–0.72) even after controlling for comorbidity and other confounding variables [36].
While tools for the evaluation of comorbidity and assessment of overall health have been previously described, there are numerous risk-stratification schemes that aid in the prediction of outcome following treatment. Specifically, D’Amico et al. evaluated the risk of biochemical recurrence 5-years after definitive therapy for prostate cancer [37] and were able to stratify patients into low-, intermediate-, and high-risk subgroups based upon clinical stage, PSA, and Gleason Score. Within this risk-stratification scheme, low-risk patients are identified as having clinical stage T1c or T2a disease, PSA ≤10, and Gleason score ≤6. Intermediate-risk patients have clinical stage T2b disease, PSA >10 but ≤20, or Gleason score 7 disease, and finally high-risk patients were defined as clinical stage T2c or greater, PSA >20, or Gleason score ≥8. These risk-groups provide a framework upon which the clinician can assess global prostate cancer risk. In addition to D’Amico’s widely utilized risk-stratification schema, there have been a number of published nomograms evaluating the risk of freedom from progression following radical prostatectomy [38], brachytherapy [39], and external beam radiation therapy (EBRT) [40]. Additionally, a model has been developed aids in the prediction of prostate cancer-specific survival following radical prostatectomy [41]. Combining these tools with previously described methods of assessment of comorbidity and overall health provides patient-specific information upon which to base treatment recommendations.
There are numerous well-described management options for a patient diagnosed with clinically organ confined prostate cancer including radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance. Radical prostatectomy (RP) involves surgical removal of the prostate and seminal vesicles, and frequently involves staging pelvic lymphadenectomy. Historically this procedure was performed using an open surgical approach through a low-midline abdominal incision. More recently, robotic-assisted laparoscopic radical prostatectomy (RALP) has been popularized secondary to potential benefits with regard to improved blood loss and improved time to convalescence [42]. Advances in open surgical technique during the 1970s and 1980s resulted in significant improvements in the morbidity associated with RP. Specifically, attention to the dorsal venous complex resulted in a reduction in operative blood loss and novel nerve-sparing techniques improved functional outcomes such as continence and erectile function [43]. Despite these improvements, RP has generally not been performed in patients over age 70 due to concern regarding the risk of perioperative mortality in patients of advanced age. The AUA guidelines identify the patient most likely to benefit from RP as having a relatively long life expectancy, no significant surgical risk factors, and a preference for surgery [44, 45]. The European Association of Urology defines RP as standard treatment in patients with stage T1b-T2b, Nx-N0, M0 disease with a life expectancy greater than 10 years. The EAU defines RP as optional in younger patients with T1a disease and a long life expectancy and in patients with limited T3a disease, Gleason score 8 or less, PSA 20 ng/mL or less, and long life expectancy [45, 46].
Few series have evaluated functional outcomes from RP in the elderly. Kerr and Zeinke reviewed the Mayo clinic experience of 51 patients older than 75 years who underwent RP and found that two-thirds of elderly patients undergoing RP were without perioperative complication. Nonetheless, elderly patients were found to have a significantly higher rate of urinary incontinence following RP than their younger counterparts (16% vs. 3%) [47]. Data from Begg et al. support the finding that chronological age appears to directly impact the risk of urinary incontinence following radical prostatectomy. The risk of perioperative death, however, was found to more strongly associate with comorbidity than chronological age [48]. There are few data that specifically address functional outcomes in elderly men following RALP. Shikanov et al., in their single-institution series, found age to be independently associated with the risk of postoperative continence and potency [49].
While there are few randomized trials which compare treatments for clinically localized prostate cancer, there is randomized data that evaluates differences in prostate cancer-related death amongst patients undergoing radical prostatectomy compared to those randomized to watchful waiting. In the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4), 695 men with clinically localized prostate cancer were randomized to undergo radical prostatectomy or watchful waiting with initiation of androgen ablation in the context of disease progression. Radical prostatectomy resulted in a relative risk of prostate cancer-related death of 0.56 (95% CI 0.36–0.88) at 10 years [50] and 0.62 (0.44–0.87) at 12 years [51], corresponding to a NNT of 15. Those patients randomized to radical prostatectomy also enjoyed more favorable rates of distant metastases, local progression, and overall survival. Nonetheless, in the subgroup of patients older than 65 years there was no discernable difference in prostate cancer-specific mortality, overall mortality, or the risk of distant metastases [50, 51]. The authors do caution that the SPCG-4 study was not powered to detect small survival within subgroups, however these data call into question the routine recommendation of radical therapy for clinically localized prostate cancer in the elderly. The application of a “one size fits all” approach, however, is not advisable considering the remarkable heterogeneity of prostate cancer, and as described above, patient-specific risk of prostate cancer death must be balanced against death from other cases.
Indeed, while the SPCG-4 study failed to reveal any difference in prostate cancer-specific mortality amongst those patients older than 65 years treated with radical prostatectomy or watchful waiting, there is data to suggest that elderly patients with high-risk disease do benefit from the addition of external beam radiotherapy to androgen ablation. The SCPG-7/SFUO-3 study randomized patients with high-risk prostate cancer to receive androgen ablation or androgen ablation plus external beam radiotherapy. At a median of 10.8 years follow-up, those patients who received radiotherapy were found to have a relative risk of death from prostate cancer of 0.44 (95% CI 0.33–0.66). The magnitude of absolute risk reduction was higher in those patients older than 67 years when compared to those younger than 67 years (12.9% vs. 9.8%, respectively) [52]. It is clear that some patients stand to benefit from definitive therapy for prostate cancer and some patients do not. The most significant challenge in treating elderly patients with prostate cancer is determining whether an individual patient is part of the former group or the latter.
EBRT remains a mainstay of treatment for clinically localized prostate cancer in the elderly. The AUA guidelines identify the patient most likely to benefit from EBRT as having a relatively long life expectancy, no significant risk factors for radiation toxicity, and a preference for EBRT [44, 45]. Additionally, the EAU guidelines recommend that treatment decision should be based upon TNM classification, Gleason score, baseline PSA, age, comorbidity, life expectancy, and health-related quality of life (HRQOL). Specific recommendations include the use of 3D-conformal radiation therapy (CRT) with or without intensity modulated radiation therapy (IMRT) for patients with T1c-T2c N0 M0 disease with dose escalation for those patients with intermediate-risk disease (T2b, PSA 10–20, Gleason score 7) [45, 46].
The question of whether one should be treated for prostate cancer revolves largely around the patient’s burden of comorbid disease and desire for treatment. Generally, the rates of biochemical control and cancer-specific survival are similar when comparing external beam radiotherapy and radical prostatectomy [53, 54]. As such, many practitioners who treat prostate cancer will base treatment recommendations upon a specific patient’s utility function(s). As previously described, there has been a tendency amongst practitioners who treat prostate cancer to not recommend surgery in patients older than 70 years secondary to increasing risks of perioperative morbidity. External beam radiotherapy is feasible in patients of advanced age, however unlike radical prostatectomy, there are no randomized trials comparing radiation therapy to expectant management. Fiorica et al. recently evaluated their experience with radiotherapy for local and locally advanced prostate cancer in patients greater than 75 years. The authors determined that patients’ burden of comorbid illness (as measured by the adult comorbidity evaluation index) was inversely related to overall survival. However, overall survival was not affected by receipt of EBRT. While comorbidity was found to strongly associate with the risk of acute bowel and urinary toxicity, there was no documented relationship between chronological age and acute or late toxicity [55]. This underscores the points raised previously in this chapter relating to the importance of careful assessment of patient comorbidity in the evaluation of both need for treatment and specific treatment choice. These data are consistent with previously published reports from pooled EORTC studies. Pignon et al. evaluated 1,619 patients who underwent radical radiotherapy for pelvic malignancy and determined that age was not associated with increasing radiation toxicity [56] thereby leading the investigators to conclude that chronologic age is not a limiting factor for radiotherapy for pelvic malignancy. These findings have been confirmed by a number of groups in a number of practice settings [57–59] leading many to conclude that radiotherapy is a reasonable treatment option for men with localized or locally advanced prostate cancer.
In addition to external beam radiotherapy, brachytherapy is a reasonable therapeutic option for men with localized low-risk disease. Unlike external beam radiation, brachytherapy is typically reserved for men with low-grade (Gleason score 6 or less) disease, minimal lower urinary tract symptoms, and small to moderate prostate volume (<50 g). In appropriately selected patients brachytherapy achieves excellent rates of biochemical progression-free survival, with cure rates approaching 99% in low-risk populations [60]. When compared to radical prostatectomy, patients undergoing brachytherapy tolerate the procedure quite well with favorable long-term quality of life outcomes [61]. Nonetheless, there are a number of problems with the general application of brachytherapy to the elderly population. Given the morbidity with regard to urinary function in men who undergo interstitial brachytherapy, many practitioners will not perform the procedure as primary prostate cancer treatment in men with significant pretreatment urinary bother (IPSS >15). Indeed, both pretreatment IPSS and prostate volume are strong predictors of posttreatment urinary toxicity in patients undergoing brachytherapy [62, 63]. Given the high prevalence of BPH and lower urinary tract symptoms in elderly men, there is considerable (and appropriate) concern over the long-term urinary morbidity of brachytherapy, which limits its general application. Furthermore, given the low-risk nature of most tumors treated by brachytherapy there is significant debate over whether or not any therapy (brachytherapy or otherwise) is indicated in elderly men with low-risk disease. Thus, while brachytherapy remains a treatment option for men with localized prostate cancer, it has not received wide acceptance in the elderly.
With the introduction and widespread dissemination of PSA screening in the early 1990s came a remarkable stage migration in prostate cancer epidemiology favoring the diagnosis of low-risk disease [21]. As discussed previously, this stage migration has generated a considerable amount of concern surrounding the overtreatment of clinically insignificant prostate tumors. Despite the nominal risk of prostate cancer-specific mortality in men diagnosed with low-risk disease, more than 90% of such men receive definitive treatment, typically in the form of surgery or radiation therapy [64]. Given the competing risks of overall mortality in the cohort of patients diagnosed with prostate cancer in contemporary series, there has been growing interest in active surveillance with curative intent, a paradigm by which patients are monitored until they demonstrate evidence of disease progression at which time definitive therapy is triggered. Active surveillance is dissimilar from watchful waiting, a paradigm by which patients are treated only should they develop symptoms often attributable to metastatic disease. Among the theoretical benefits of active surveillance is a significant reduction in prostate cancer overtreatment, particularly in elderly men who stand to benefit little from radical therapy.
Active surveillance series are beginning to mature, and while follow-up remains short- to intermediate-term, these series do show promising results. Enrollment criteria for the Johns Hopkins Active Surveillance program includes patients with clinical stage T1c disease, PSA density less than 0.15 ng/mL, Gleason score 6 or less, two or fewer biopsy cores with cancer, and a maximum of 50% involvement of any core with cancer [65]. The investigators have followed a total of 769 men with a median follow-up of 2.5 years and found that a total of 33% of men underwent intervention at a median time of 2.2 years with 73.7% of these men doing so for biopsy reclassification. Nonetheless, there were no men in the Johns Hopkins Program who developed distant metastatic disease or died from prostate cancer [66]. Perhaps the most mature active surveillance cohort is from the University of Toronto, where the surveillance cohort is somewhat more heterogeneous. Enrollment criteria in the Toronto program currently include all low-risk patients (Gleason score 6 or less and PSA less than 10 ng/mL); however, from 1995 to 1999 the program did enroll patients older than 70 years with PSA up to 15 ng/mL or Gleason 3+4 disease. Investigators have followed 450 patients for a median of 6.8 years and reported a treatment rate of 30%. The 5- and 10-year cancer-specific survival in the cohort was 99.7% and 97.2%, respectively, with a hazard ratio for other-cause mortality of 18.6 (95% CI 7.6–45.7) [67].
While the risk of prostate cancer-specific mortality seems low compared to other competing causes of death, particularly in patients with a moderate to high burden of medical comorbidity, there remains some concern about missed opportunity for intervention. Warlick et al. evaluated the risk of “noncurable” cancer in patients undergoing immediate radical prostatectomy compared to delayed surgery after having been enrolled in an active surveillance program. Using a definition of “noncurable” cancer as adverse pathology associated with a less than 75% chance of remaining disease-free 10 years after surgery, the investigators found that, after adjustment for PSA density and age, there was no difference in the risk of “noncurable” cancer between those treated immediately and those undergoing delayed intervention [68]. This data indicates that there is little risk in enrollment in active surveillance with delayed intervention. There remains some controversy, however, regarding the safety of delayed treatment. O’Brien et al. evaluated patients with D’Amico low-risk disease and determined that those patients who suffered a treatment delay of 6 months or greater were found to have higher risk of pathologic upgrading and biochemical failure after prostatectomy. Surgical delay remained an independent predictor of biochemical failure on multivariable analysis when controlling for PSA and clinical stage [69]. Thus, while active surveillance appears to be a safe management strategy for many men with low-risk prostate cancer, long-term follow-up will be required to carefully assess the survival implications associated with delayed intervention. The elderly population, particularly elderly patients with low-risk disease, should certainly be counseled regarding the merits and pitfalls of active surveillance.
The diagnosis and management of elderly patients with prostate cancer remains a challenge to treating physicians. Unlike many other malignancies, the risk of diagnosis and treatment must be carefully considered in the context of competing risks of death. Working groups have begun to develop paradigms for prostate cancer management in the elderly. Utilization of prediction tools coupled with the use of such management paradigms (Fig. 9.1, [94]) will help clinicians and patients alike achieve optimal treatment strategies. While current prediction tools remain imperfect, they are helpful in the quantification of risk when counseling such patients. As data from ongoing studies begins to mature, we will continually have to reassess the paradigm of prostate cancer screening, diagnosis, and treatment.
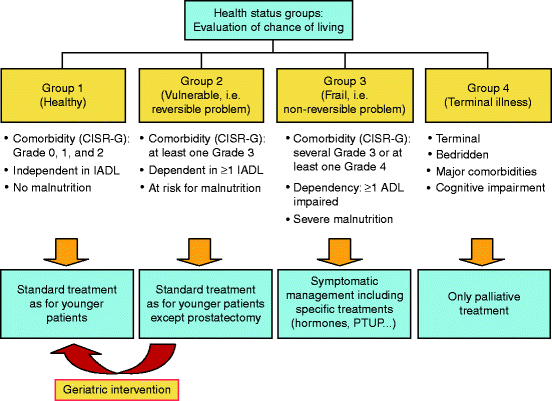

Fig. 9.1
A decision tree for treating patients with localized disease and metastatic disease
Bladder Cancer
Introduction and Epidemiology
Bladder cancer is a commonly diagnosed malignancy in both men and women, accounting for over 70,000 incident cases and over 14,000 deaths in 2010 [12]. Like many other malignancies, bladder cancer occurs most commonly in the elderly, with the median age at diagnosis being 69 and 71 years in men and women, respectively [70]. Indeed, the incidence of bladder cancer seems to increase with increasing age. Schultzel et al. evaluated the California Cancer Registry and determined that the incidence of bladder cancer peaks in those patients 85 years and older regardless of sex or ethnic background [71]. Interestingly, these investigators documented a 10 year difference in peak incidence between lung/bronchus cancer and bladder cancer despite similar risk factors such as smoking and occupational exposure [71]. Nonetheless, there is a strong association of bladder cancer incidence with age, with an overall incidence of 28.6% in patients under 65 years and 71.4% in those over 65 years [3].
Bladder cancer treatment and outcome is largely determined by disease grade and stage. While nonmuscle-invasive tumors are frequently managed with transurethral resection and intravesical immunotherapy/chemotherapy, muscle-invasive tumors require radical therapy traditionally in the form of radical cystectomy and urinary diversion. Given the significant difference in treatment strategy amongst patients with either nonmuscle-invasive or muscle-invasive disease, there is considerable interest in identifying relationships that exist between chronologic age and disease aggressiveness. Indeed, it seems as though there exists a direct relationship between age and the risk of diagnosis of muscle-invasive bladder cancer. Konety et al., using SEER data, found that the risk of muscle-invasive bladder cancer increases to 32.4% in those patients 85 years or older from 23.8% in those 55–64 years [72, 73]. This relationship was confirmed by Prout et al. who, using similar data, found the risk of muscle-invasive disease to be 28.7% in those patients 85 years or older compared to 19.4% in those less than 55 years [73, 74].
Nonmuscle-Invasive Bladder Cancer: Treatment Implications in the Elderly
As described above, the hallmarks of treatment for nonmuscle-invasive bladder cancer are transurethral resection and intravesical immunotherapy/chemotherapy. In the group of elderly patients with nonmuscle-invasive disease, endoscopic procedures including transurethral resection are generally well tolerated. Transurethral resection rarely creates scenarios in which major fluid shifts or pressures are transmitted to the cardiopulmonary, renal, or hepatic systems [3]. Generally, patients with low-grade, nonmuscle-invasive disease face little risk with regard to disease progression. Specifically, the risk of progression in patients with low-grade Ta disease is approximately 5–10% [75]. Emphasis is placed on local disease control, with particular attention paid to limiting the morbidity associated with such tumors. Patients with high-grade tumor(s), invasive disease, or carcinoma-in-situ, however, face higher risk of disease progression ranging from 15% to >50% [75] depending on the specific stage and grade of the tumor of interest. Traditionally, transurethral resection in combination with intravesical immunotherapy and/or chemotherapy has been used to reduce both disease recurrence and progression. Bacillus Calmette-Guerin (BCG) is the most common form of intravesical therapy used in the United States. Intravesical administration of BCG results in a local immune response with cytokine induction favoring interferon-γ and IL-2. Such response is thought to activate cell-mediated cytotoxic mechanisms that translate into disease control [75, 76].
The efficacy of intravesical BCG in reducing the likelihood of disease recurrence [75, 77–79] and progression [75, 80, 81] has been well described in the literature. Nonetheless, there is a growing body of evidence to suggest that the response to BCG is attenuated in the elderly. Joudi et al. evaluated data from a national phase II multicenter study of BCG plus interferon-alpha to assess differences in response to treatment based upon age. Investigators found the 2-year recurrence-free survival rates in patients 80 or older and 61–70 years to be 39% and 61%, respectively. The response rates [82] amongst elderly patients were lower than younger patients regardless of whether these patients were BCG-naïve or had been previously treated [83]. Herr reviewed the Memorial Sloan Kettering experience and found that, while there was no difference recurrence-free survival at 2 years, after 5 years, those patients older than 70 years demonstrated less favorable recurrence-free survival when compared to those younger than 70 years (27% vs. 37%, respectively) [84]. It is known that both innate and adaptive immunity declines with age [3, 85] and as such, it is theorized that elderly patients may not be able to mount the immune response necessary for optimal BCG response. Nonetheless, the magnitude of age as a negative predictor of response to intravesical BCG likely remains fairly small.
While the response to BCG appears to be somewhat attenuated with advancing age, there is some evidence to suggest that morbidity from treatment increases with advancing age. Heiner and Terris reviewed their experience of men receiving maintenance BCG and found the complication rate for patients less than 70 years and greater than 70 years to be 17.6% and 48.6%, respectively [86]. The most common complications reported in this series included cystitis/bladder pain severe enough to discontinue therapy, chills/fever, and severe prostatitis/epididymitis. These data led to the authors to conclude that maintenance should be given with caution in men over age 70 and should be avoided in patients over age 80 [86]. There are few other series that specifically address the risk of complication from intravesical BCG in the elderly; however, there are a number of case reports that raise concern over this association [82, 87, 88]. Clearly, the benefits of intravesical BCG with regard to reduction in the risk of disease recurrence and progression must be weighed against the risks of BCG therapy in patients of advanced age.
Muscle-Invasive Bladder Cancer: Treatment Implications in the Elderly
The gold standard treatment for muscle-invasive bladder cancer is radical cystectomy (RC) and urinary diversion [89]. Furthermore, recent data supports the use of neoadjuvant platinum-based chemotherapy in patients with muscle-invasive bladder cancer [90–92]. Nonetheless, radical surgery and systemic chemotherapy confer significant “stress” to a number of organ systems, and given the gradual decline in physiologic capacity among the elderly, each of these interventions is limited by the patients’ abilities to tolerate the insult(s) of treatment [3]. Indeed, patients aged 75 years or older have, not surprisingly, a higher prevalence of cardiac disease prior cancer diagnosis, chronic anemia, and less favorable American Society of Anesthesiologists (ASA) Classification than those less than 75 years [74].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







