Urinary tract infection (UTI) is a frequent diagnosis in children who are referred to the urologist. Although most infections will resolve without complication after appropriate treatment, a wide array of potential complicating factors exists, which can make difficult the rapid resolution of a UTI. Clinical scenarios involving these factors require a high index of suspicion and prompt initiation of appropriate therapy.
Urinary tract infection (UTI) is a significant concern for parents as well as for children who acquire them. While primary care physicians and pediatricians are the front line, dealing with the initial management of UTI, they turn to urologists when faced with more complicated infections. This article reviews the diagnosis and management of UTI, and examines scenarios in which the clinician should have a heightened level of concern when dealing with UTI in the pediatric population.
The comprehensive epidemiology of UTI has been well described. The overall incidence of UTI in the prepubertal pediatric population is 3% in girls and 1% in boys. The incidence of UTI varies with age and sex. Infant girls have an incidence of 0.4% to 0.1%, which increases to 0.9% to 1.4% between the ages 1 and 5 years, and peaks with an incidence of 0.7% to 2.3% in school-aged girls. In contrast, infant boys have an incidence of UTI of 0.188% (circumcised) and 0.702% (uncircumcised), which decreases to 0.1% to 0.2% between ages 1 and 5 years, followed by 0.04% to 0.2% in school-aged boys. In febrile children presenting to the emergency department, UTI is more common than in healthy children, with an incidence between 3% and 5% in most studies. Racial differences also exist, including a low incidence in African American children and higher incidence in Caucasian girls relative to other races. Other risk factors associated with UTI include anomalies of the urinary tract (anatomic, functional, or neurologic) and systemic abnormalities (diabetes mellitus, compromised immune system, and so forth).
The pathogenesis of UTI is based both on the bacteria that cause infection and on patient-specific factors. Bacteria common in UTI are predominantly of enteric origin. Escherichia coli is the most frequent cause of all types of UTI, while group B streptococcal infection is relatively more common in neonates. Bacteria tend to colonize the periurethral area, migrating in a retrograde fashion to reach the urinary tract. Bacteria may also be introduced into the urinary tract via instrumentation. Systemic infections may also result in UTI through seeding of the urinary system. Once present within the urinary tract, bacteria can be cleared by the emptying of urine or can adhere to the urothelial lining, resulting in infection. After colonization of the urinary tract, virulence factors such as fimbriae may assist bacteria in causing an infection.
Diagnosis of UTI is based on clinical symptoms and the results of a urine culture. Classic symptoms of UTI in adults are dysuria, frequency, hesitancy, and flank pain. Unfortunately, young children often lack the ability to identify and describe these symptoms. Symptoms in children tend to be less specific in nature and parents commonly report their symptoms as fever, irritability, lethargy, poor feeding, incontinence, and pungent urine odor. Children presenting with these symptoms, or with unexplained fever, should have UTI eliminated as a diagnosis. On performing a genitourinary examination, no specific abnormalities are consistently present. Definitive diagnosis of a UTI requires a properly obtained urine culture. Perineally “bagged” urine is useful only for excluding UTI, as there is a high chance that any growth is the result of skin colonization. Clean catch urine specimens also have a higher false-positive rate in young children, likely due to periurethral colonization. This collection technique can be more useful in older children.
The 2 most reliable sources of urine for culture are a catheterized urine specimen or a suprapubic aspirate. The drawbacks of catheterized urine include the potential for (a) introduction of bacteria, which may lead to an iatrogenic infection, and (b) psychological trauma to the patient. Suprapubic aspirates avoid introduction of pathogens into the urinary tract and give a reliable specimen; however, the use of this technique is limited by physician comfort. A suprapubic aspirate is obtained by blind passage of a small-gauge (21F or 22F) needle through the abdominal wall approximately 1 to 2 cm cephalad to the pubic symphysis into a bladder that is palpably full. The use of bedside ultrasonography enhances the ability to safely perform this technique by ensuring an adequately full bladder and allowing assessment of structures between the abdominal wall and bladder, while topical anesthesia can decrease patient discomfort.
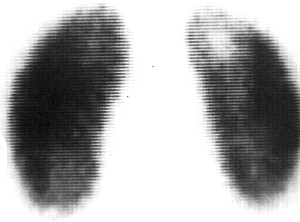
Imaging for UTI is a subject of ongoing debate beyond the scope of this article. Prior teaching deferred imaging for a nonfebrile UTI, while recommending renal/bladder ultrasonography and voiding cystourethrography (VCUG) for a febrile UTI. More recently, the top-down approach has been advocated as a method of avoiding VCUG and concentrating effort on those at greatest risk of renal scarring. This approach, which will be discussed in detail elsewhere in this issue, focuses on using a dimercaptosuccinic acid (DMSA) scan to document pyelonephritis and/or renal scarring. Additional lower tract imaging with a VCUG is performed on patients with documented renal involvement. DMSA scans are considered the gold standard for detection of acute pyelonephritis and renal scarring ( Fig. 1 ), with 92% sensitivity when compared with histology in an animal model. As always, limitation of radiation exposure is a goal in pediatrics, and use of imaging modalities that limit radiation exposure while providing the necessary details will continue to increase as techniques improve. Magnetic resonance imaging (MRI), including MR urography, has increasingly been used to image the urinary tract and to identify renal scarring, with some literature questioning whether MRI is superior to DMSA. While these studies avoid radiation exposure, they often require sedation or general anesthesia when performed in children. Renal/bladder ultrasonography (RBUS) is less sensitive for detecting pyelonephritis than DMSA scan, with one study noting only 22% detection of DMSA-confirmed scarring using RBUS. Ultrasound, however, does allow for assessment of renal abnormalities, such as hydronephrosis and duplication anomalies, as well as monitoring of renal growth and the presence of parenchymal thinning; it does so without the need for sedation or anesthesia, and without ionizing radiation exposure.
Classification of UTI has been complicated by the number of prior systems that have been used. Classification by site of infection prompts designation as cystitis (bladder infection) or pyelonephritis (kidney infection). Unfortunately, clinical symptoms alone do not always accurately differentiate upper from lower tract UTI. Previous work using ureteral catheterization to localize the site of infection demonstrated upper tract bacteria in less than 50% of those with fever and flank pain, but in 20% of asymptomatic patients. Infections can also be designated as uncomplicated versus complicated, or initial versus recurrent.
Recurrent UTI may be further categorized as unresolved bacteriuria, bacterial persistence, or reinfection. Unresolved bacteriuria results from inadequate treatment of a known pathogen. Persistence implies appropriate treatment of the UTI, but persistence of the infecting organism within a nidus of infection or within an area that is isolated from treatment. On immediate posttreatment urine culture the same bacterial pathogen will quickly return. Reinfection requires repeated UTI with different bacteria, which may include different bacterial serotypes and clones. The importance of differentiating between persistence and reinfection is that persistence may be surgically correctable.
Treatment of UTI
Treatment of UTI focuses on the site of infection, presence of fever, and the pathogen causing the infection. Ampicillin and gentamicin continue to be the mainstay of empirical treatment of pyelonephritis. The use of a third-generation cephalosporin may be considered with the knowledge that its coverage will not include Enterococcus and that there is emerging extended-spectrum β-lactam resistance. When a patient has recently been on antibiotics, it is worthwhile to consider using alternative choices due to the possibility of resistant bacteria. Once afebrile for 24 to 48 hours, consideration can be given to transitioning to oral (PO) antibiotics. Improvement in serum markers such as the white blood cell count or C-reactive protein is also encouraging when considering transition to oral antibiotics. The use of longer duration of intravenous (IV) antibiotics has not been shown to be superior to an early transition to PO therapy in preventing scarring based on DMSA scans at 9 months. In all cases, the combination of IV and oral therapy should include 10 to 14 days of appropriate antibiotics, with neonates and more severe infections favoring the longer duration.
Although traditional teaching has been that febrile UTI should be treated promptly with IV antibiotics in an inpatient setting to avoid renal scarring, recent data have brought this teaching into question. In a study by Hewitt and colleagues from Italy, the frequency of renal scarring on DMSA scan at 1 year was similar (approximately 30%) in those treated early in a comparison with treatment by a delayed fashion. Nonetheless, treatment should be started as soon as possible to relieve symptoms and with the hope of avoiding renal scarring. Even with upper tract involvement, outpatient treatment of UTI has been shown to be safe and effective, particularly in older children who are tolerating oral intake and are clinically stable. In these cases, outpatient treatment with trimethoprim/sulfamethoxazole (TMP/SMX), cephalosporins, or fluoroquinolones are viable options. Nitrofurantoin is inadequate when renal involvement is suspected as a result of poor tissue levels. In addition, daily intramuscular (IM) injection of a once-a-day broad-spectrum antibiotic (such as ceftriaxone) is an option. This treatment should be continued either until identification/sensitivities can direct oral therapy, or for the entire outpatient course when more convenient than parenteral antibiotics using a peripherally inserted central catheter. A conservative approach of hospital admission for IV antibiotics is justified when the clinical picture, social scenario, or patient age (particularly neonates) dictates. In these cases, IV rehydration and broad-spectrum antibiotics are administered.
Cystitis in children can safely be treated using nitrofurantoin, sulfonamides, TMP/SMX, trimethoprim alone, and cephalosporins. In addition, ciprofloxacin is also used in children, for whom it is approved as a second-line therapy in complicated UTI. Use of ciprofloxacin in children is reserved for more serious cases due to concerns over potential cartilage damage. Fortunately, in studies of children who received ciprofloxacin, complications have been reversible after discontinuation. Most often, TMP/SMX or nitrofurantoin is a good initial therapy for uncomplicated cystitis until final urine culture and sensitivities have returned. Regional resistance to TMP/SMX is known, and this should be taken into account in the decision to use TMP/SMX as initial therapy. Once final sensitivities are reported, treatment should be adjusted to ensure appropriate antibiotic coverage of the infecting organism. The addition of an IM antibiotic dosage has not been shown to be of significant benefit in febrile UTI and its usage in cystitis is likely not warranted. Duration of treatment is largely age based in this population. A 3-day course is adequate in the clinically stable child with uncomplicated cystitis, while longer treatment courses (7–10 days) are likely appropriate for children younger than 2 years. Although a recent study from Canada has shown feasibility of outpatient ambulatory treatment with parenteral antibiotics in 1- to 3-month old children with febrile UTI, the very young and those who are dehydrated, unable to tolerate oral medications, or toxic appearing warrant a conservative approach with admission for parenteral antibiotics and hydration.
When to worry less
It is important for clinicians to be familiar with situations in which there is a relatively low risk for patients. These scenarios can be perplexing for parents and primary care physicians who do not encounter such urologic scenarios on a consistent basis. For example, urine cultures growing Lactobacillus species, coagulase-negative staphylococci, and Corynebacterium species are not considered pathogens in otherwise healthy children of 2 months to 2 years old, and treatment is unnecessary.
During the period of toilet-training, children are at an increased risk of lower UTI because of changes in voiding and stooling habits. Less than optimal hygiene, in combination with the newly developed ability to hold one’s urine, can lead to UTIs. While still warranting treatment, these infections may be more related to functional changes. In the case of an isolated UTI during toilet-training, establishing good voiding and stooling habits is the primary goal after initial treatment of the UTI.
The presence of a UTI in the setting of corrected or spontaneously resolved reflux can cause significant anxiety for parents and primary care physicians, while not posing as great a risk as perceived. After the initial diagnosis of vesicoureteral reflux (VUR), parents often become conditioned to associate UTI and the risk of damage to the kidneys. The correction of VUR does not decrease the risk of a child developing a lower UTI but only eliminates the reflux of infected urine into the kidney, thereby preventing or delaying the development of upper UTI. It is important to ensure that parents understand the purpose of VUR correction, are informed that VUR correction does not alter host susceptibility to UTI, and are counseled to seek appropriate treatment for UTI.
Finally, a clinical scenario that is challenging to understand is asymptomatic bacteriuria. Clinical situations exist in which colonization of the urinary tract is inevitable. In these situations, the presence of bacteria is normal and does not require treatment despite a positive urine culture. Examples of scenarios in which the urinary tract can be expected to be colonized are patients with long-term indwelling tubes, patients performing clean intermittent catheterization (CIC), patients with intestinal neobladders or augmented bladders, and patients in whom the urinary tract is opened to the skin (vesicostomy, ureterostomy, and so forth). In these cases, routine bacteria cultured from the urinary tract and not causing significant clinical symptoms (dysuria, incontinence, fever, and so forth) should not be treated. One should also favor observation for bacteria noted on a screening urinalysis performed in an asymptomatic patient without complicating factors. Treatment of these asymptomatic bacteria will only allow recolonization with different, potentially more pathogenic bacteria and increase the risk of antibiotic resistance. Fever in a setting of asymptomatic bacteriuria should be worked up as a fever of unknown origin, including urine culture and blood cultures, with treatment as a UTI reserved for cases in which another source is not identified. Pyuria on a concurrent urine analysis can aid in confirming the diagnosis of clinical UTI. While the aforementioned situations are examples of times when excessive concern is not warranted, one should always use common sense when approaching these issues. When additional symptoms, repeated infections, or a confusing clinical scenario presents, further investigation and an increased clinical index of suspicion for the presence of more serious urologic issues is always reasonable.
When to worry less
It is important for clinicians to be familiar with situations in which there is a relatively low risk for patients. These scenarios can be perplexing for parents and primary care physicians who do not encounter such urologic scenarios on a consistent basis. For example, urine cultures growing Lactobacillus species, coagulase-negative staphylococci, and Corynebacterium species are not considered pathogens in otherwise healthy children of 2 months to 2 years old, and treatment is unnecessary.
During the period of toilet-training, children are at an increased risk of lower UTI because of changes in voiding and stooling habits. Less than optimal hygiene, in combination with the newly developed ability to hold one’s urine, can lead to UTIs. While still warranting treatment, these infections may be more related to functional changes. In the case of an isolated UTI during toilet-training, establishing good voiding and stooling habits is the primary goal after initial treatment of the UTI.
The presence of a UTI in the setting of corrected or spontaneously resolved reflux can cause significant anxiety for parents and primary care physicians, while not posing as great a risk as perceived. After the initial diagnosis of vesicoureteral reflux (VUR), parents often become conditioned to associate UTI and the risk of damage to the kidneys. The correction of VUR does not decrease the risk of a child developing a lower UTI but only eliminates the reflux of infected urine into the kidney, thereby preventing or delaying the development of upper UTI. It is important to ensure that parents understand the purpose of VUR correction, are informed that VUR correction does not alter host susceptibility to UTI, and are counseled to seek appropriate treatment for UTI.
Finally, a clinical scenario that is challenging to understand is asymptomatic bacteriuria. Clinical situations exist in which colonization of the urinary tract is inevitable. In these situations, the presence of bacteria is normal and does not require treatment despite a positive urine culture. Examples of scenarios in which the urinary tract can be expected to be colonized are patients with long-term indwelling tubes, patients performing clean intermittent catheterization (CIC), patients with intestinal neobladders or augmented bladders, and patients in whom the urinary tract is opened to the skin (vesicostomy, ureterostomy, and so forth). In these cases, routine bacteria cultured from the urinary tract and not causing significant clinical symptoms (dysuria, incontinence, fever, and so forth) should not be treated. One should also favor observation for bacteria noted on a screening urinalysis performed in an asymptomatic patient without complicating factors. Treatment of these asymptomatic bacteria will only allow recolonization with different, potentially more pathogenic bacteria and increase the risk of antibiotic resistance. Fever in a setting of asymptomatic bacteriuria should be worked up as a fever of unknown origin, including urine culture and blood cultures, with treatment as a UTI reserved for cases in which another source is not identified. Pyuria on a concurrent urine analysis can aid in confirming the diagnosis of clinical UTI. While the aforementioned situations are examples of times when excessive concern is not warranted, one should always use common sense when approaching these issues. When additional symptoms, repeated infections, or a confusing clinical scenario presents, further investigation and an increased clinical index of suspicion for the presence of more serious urologic issues is always reasonable.
When to worry
The authors now focus attention on situations in which UTI is more complicated, often requiring a high index of clinical suspicion and a low threshold to proceed to admission, broad-spectrum antibiotics, further investigation, and pediatric urology consultation. Attempts have been made to sort these infrequent scenarios into more generalized groups; however, many pathologic processes could be placed under multiple headings. The rare nature of very complicated UTI makes research comparing different approaches to treatment difficult. Prospective placebo-controlled studies do not exist. In these complex cases, there are undoubtedly multiple effective ways to approach treatment. When the literature does not provide clear evidence supporting one approach, information is provided on the clinical pathway followed by the authors for managing these difficult situations.
Some general principles apply in these complex clinical scenarios. The presence of abnormal anatomy, particularly abnormal drainage, should always prompt additional workup in the presence of UTI. The presence of prior renal scarring should also prompt additional concern, as these patients are starting with fewer functioning nephrons and have established they are susceptible to renal injury. Failure of a patient to respond to conventional treatment of a UTI should also prompt concern. Additional workup should be performed to confirm that culture-specific antibiotics are being used, that adequate drainage exists, and that the antibiotics reach all sites of bacterial infection.
Bad Pathology
While a single febrile UTI is a cause for concern, the presence of repeated febrile infections should alert all physicians to the need for a more extensive evaluation. One must be concerned about the presence of a physiologic or anatomic patient factor as the origin. While most renal scarring is felt to occur with the first episode of pyelonephritis, the “big bang theory,” recurrent pyelonephritis can cause increased renal scarring. A comprehensive workup, with special focus on voiding and bowel habits, family history of recurrent UTI, and activities preceding the infections, should be undertaken. Urine culture results should be reviewed to assess for evidence of bacterial persistence. If true persistence exists, further imaging should be performed to evaluate for a source of the bacteria. Renal bladder ultrasonography and voiding cystourethrography will allow one to quickly assess the upper and lower tract anatomy while minimizing radiation exposure. Additional imaging may be required based on the clinical situation. In a toilet-trained child, a urinary flow rate and postvoid residual should be obtained to assess bladder emptying. Consideration should be given to antibiotic prophylaxis.
Pyonephrosis and emphysematous pyelonephritis are 2 severe infections of the kidney. Pyonephrosis is the presence of purulence and sediment within the renal collecting system. Presenting with a picture similar to pyelonephritis, these patients may not have resolution with antibiotics alone because of the presence of obstruction. In children most pyonephrotic kidneys are nonfunctional or have very poor function. Treatment always involves broad-spectrum antibiotics and frequently drainage of the collecting system, either via retrograde stent or nephrostomy tube placement. Emphysematous pyelonephritis is an infection with air seen in the collecting system on imaging. This entity is extremely rare in children. Percutaneous drainage and antibiotics should be considered first-line therapy. Nephrectomy, which was previously considered the treatment of choice, should be reserved for those who do not respond to conservative management.
Renal abscesses ( Fig. 2 ) have become relatively rare in the pediatric population since the advent of modern antimicrobial drugs. These infections may develop via ascending infection, in which case the offending organism will be those seen in UTI ( E coli and so forth) or via hematogenous spread, in which case staphylococcal infection is more common. Focal bacterial nephritis (acute lobar nephronia) is an acute form of bacterial nephritis affecting 1 or more renal lobules, with some series demonstrating up to 25% progression to abscess. Symptoms associated with abscesses are often those of severe pyelonephritis. Abscesses of 3 cm or less respond well to conservative management with antibiotics and observation in patients with normal urinary tracts and immune systems. Surgical drainage of the kidney was historically the gold standard of care; however, more recently percutaneous drainage using computed tomography (CT) or ultrasound guidance has been found to be effective. In either situation sampling of the abscess fluid with aerobic, anaerobic, and fungal cultures should be performed to assist in care. A single percutaneous drainage procedure may be adequate with smaller abscesses, whereas very large abscesses may warrant placement of a drain to both avoid reaccumulation and facilitate antibiotic penetration. Broad-spectrum antibiotics should be employed, initially guided by urine or blood culture results, then by culture of the abscess fluid. When a urinary tract source is suspected, ampicillin and gentamicin remain good first options, whereas an extended spectrum penicillin or cephalosporin is a good first choice when a hematogenous source is suspected. Follow-up imaging to confirm resolution of the abscess should be obtained.







