Urinary Cytology
The use of urine in the medical field dates back to the ancient Egypt, but it was not until the 19th century that Lambl described the first microscopic evaluation of urine cytology in 1856. The use of urine cytology as a screening and diagnostic tool started gaining acceptance after the description of the urine sediment by Papanicolaou in 1945 (1). Since then, there has been extensive literature on the urine cytology in the management of urothelial carcinoma of the bladder. Despite this fact, the relative clinical value of the test remains controversial. Urine cytology remains poorly understood by most urologists and difficult for many pathologists. The literature documents the low sensitivity of urine cytology as a whole but rarely makes note of the fact that sensitivity is grade-dependent. One has to keep in mind that the large majority of urothelial carcinomas are low-grade carcinomas, leading to the bias of sensitivity. The fact is that urine cytology should be used mainly for diagnosis and follow-up of high-grade urothelial carcinoma, whether flat [carcinoma in situ (CIS)], papillary or mixed (2). It is not infrequent to find a case of flat urothelial CIS in which the urine cytology is positive while the cystoscopy and biopsy are negative with the diagnosis being confirmed a few months later. In experienced hands, the specificity of a positive urine cytology approaches 100% particularly for high-grade lesions.
URINE SPECIMENS: SPECIMEN TYPES AND NORMAL FINDINGS
There are three main types of urine that can be used for cytologic evaluation, voided, instrumented, and ileal conduit. The specimens should be processed immediately or refrigerated, and then processed as rapidly as possible. Alternatively, the specimen should be immediately fixed in an equal volume of 50% ethanol or preferably centrifuged, and the sediment mixed with an ethanol- or methanol-based fixative for liquid-based cytology, such as Cytolyt. Standard criteria for adequacy assessment have not been established, but the report should include information such as low cellularity, poor preservation, obscuring blood, or inflammation (3).
Voided Urine
Voided urine is the easiest way to obtain urine specimens, and its main advantage is that it is a noninvasive test. The preferred sample should
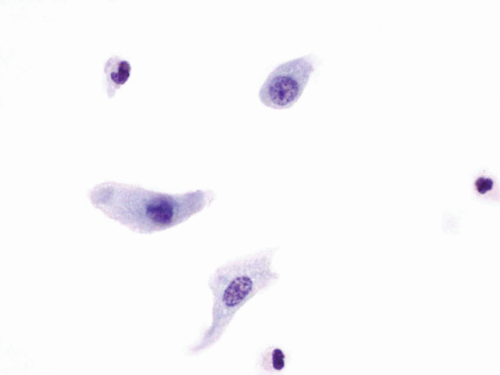
come from the second morning midstream urine specimen after hydration. Three second “morning voided” urines collected over three consecutive days appears to optimize the detection of urothelial malignancies (4). The specimens are usually paucicellular and composed of urothelial cells (Fig. 14.1), umbrella cells, and squamous cells, which can be admixed with inflammatory cells or red blood cells. The umbrella cells mostly have one nucleus, although multinucleated forms can be seen. They have abundant delicate cytoplasm, resulting in a low N/C ratio, and the nuclei have smooth nuclear contours with fine chromatin pattern and a small chromocenter (Fig. 14.2). The cytoplasm can be occasionally vacuolated. Excluding
the umbrella cells, the other urothelial cells originate from the basal and intermediate layers, and they have a columnar or cuboidal appearance. These cells are smaller than the umbrella cells and display less cytoplasm. The nuclei are round with smooth nuclear contours, and the chromatin pattern is fine. Nucleoli are either absent or single and minute. Clusters of basal and intermediate urothelial cells can be seen in reactive conditions such as urolithiasis, postinstrumentation, but the presence of a papillary cluster with a vascular core should raise the possibility of a low-grade papillary lesion.
come from the second morning midstream urine specimen after hydration. Three second “morning voided” urines collected over three consecutive days appears to optimize the detection of urothelial malignancies (4). The specimens are usually paucicellular and composed of urothelial cells (Fig. 14.1), umbrella cells, and squamous cells, which can be admixed with inflammatory cells or red blood cells. The umbrella cells mostly have one nucleus, although multinucleated forms can be seen. They have abundant delicate cytoplasm, resulting in a low N/C ratio, and the nuclei have smooth nuclear contours with fine chromatin pattern and a small chromocenter (Fig. 14.2). The cytoplasm can be occasionally vacuolated. Excluding
the umbrella cells, the other urothelial cells originate from the basal and intermediate layers, and they have a columnar or cuboidal appearance. These cells are smaller than the umbrella cells and display less cytoplasm. The nuclei are round with smooth nuclear contours, and the chromatin pattern is fine. Nucleoli are either absent or single and minute. Clusters of basal and intermediate urothelial cells can be seen in reactive conditions such as urolithiasis, postinstrumentation, but the presence of a papillary cluster with a vascular core should raise the possibility of a low-grade papillary lesion.
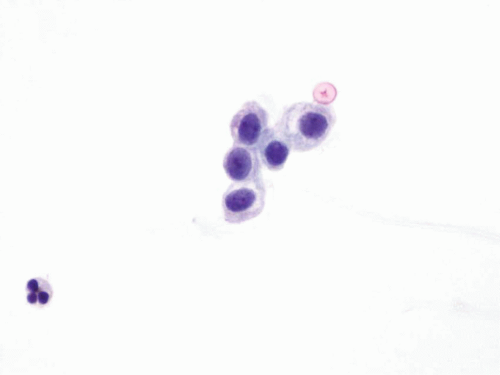 FIGURE 14.1 Benign urothelial cells: Small cells with fine chromatin pattern and smooth nuclear contours. |
Instrumented Urine
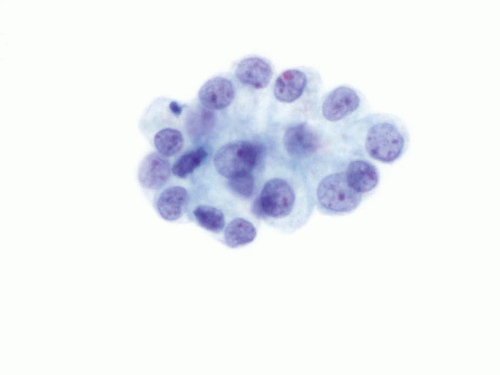
Urine obtained through catheterization of the bladder is more cellular and contains umbrella cells, urothelial cells from the basal and intermediate layer, squamous cells, and red blood cells. These specimens commonly show the presence of clusters of urothelial cells forming cell balls and pseudopapillary clusters. In general, the urothelial cells have low N/C ratio, round nuclei, fine chromatin pattern, and smooth nuclear contours (Fig. 14.3). However, instrumentation effect might be present and induce a false positive diagnosis. The instrumentation effect can be noted in the form of crowded cluster formations, hyperchromasia, and distinct nucleoli (Fig. 14.4). These cytologic features might be more evident in instrumented urine specimens because the cells in these specimens are usually well preserved.
Ileal Conduit Urine
Ileal conduit or neobladder are the most common urine diversion techniques used in patients who have undergone cystectomy. Both techniques
use a portion of the ileum to anastomose the ureters to the skin or to the urethra. The cytology specimens from these specimens are cellular with the presence of many degenerated single cells and few glandular cells in a dirty background. The degenerated cells have an oval shape, but often pyknotic nuclei, while the better preserved glandular cells display vacuolated cytoplasm and hyperchromatic nuclei with preserved N/C ratio. Nonspecific eosinophilic cytoplasmic inclusions are usually present (5) (Fig. 14.5). Ileal conduit urine can be a potential pitfall in the evaluation of urine specimens
as the hyperchromasia present in degenerated cells might lead to a false positive diagnosis. A diagnosis of malignancy in an ileal conduit requires the presence of highly atypical cells with irregular nuclear contours, coarse chromatin, and high N/C ratio.
use a portion of the ileum to anastomose the ureters to the skin or to the urethra. The cytology specimens from these specimens are cellular with the presence of many degenerated single cells and few glandular cells in a dirty background. The degenerated cells have an oval shape, but often pyknotic nuclei, while the better preserved glandular cells display vacuolated cytoplasm and hyperchromatic nuclei with preserved N/C ratio. Nonspecific eosinophilic cytoplasmic inclusions are usually present (5) (Fig. 14.5). Ileal conduit urine can be a potential pitfall in the evaluation of urine specimens
as the hyperchromasia present in degenerated cells might lead to a false positive diagnosis. A diagnosis of malignancy in an ileal conduit requires the presence of highly atypical cells with irregular nuclear contours, coarse chromatin, and high N/C ratio.
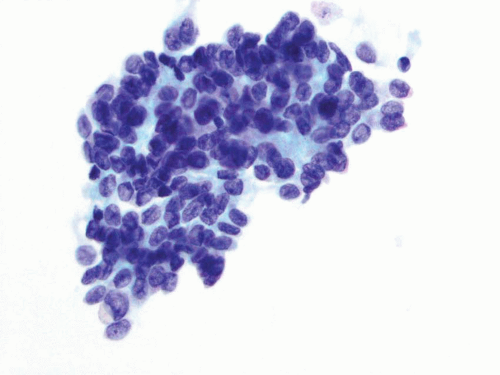 FIGURE 14.3 Instrumented urine: Clusters of benign urothelial cells with low N/C ratio, round nuclei, fine chromatin pattern, and smooth nuclear contours. |
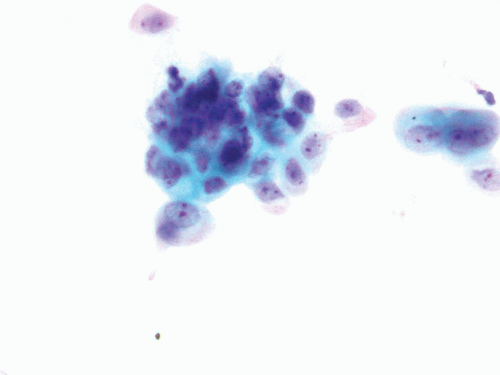 FIGURE 14.4 Instrumentation reactive atypia: Crowded cluster formations, hyperchromasia, and distinct nucleoli. |
NON-NEOPLASTIC CONDITIONS
Infection
Bacterial cystitis affects predominantly women in the reproductive age group. The findings in the urine are nonspecific. The specimens usually contain abundant neutrophils and few urothelial cells, which are enlarged with vacuolated cytoplasm. The nuclear size of the urothelial cells might range from small to large but the cells maintain their N/C ratio and the chromatin pattern is still fine. Urothelial cells with degenerative changes can be seen.
Human Polyomavirus Infection
Infection by the polyomavirus is usually due to a reactivation of the virus in immunosuppressed patients. The immunosuppression might be caused by chemotherapy, diabetes, organ transplantation, or AIDS (6). Infection by polyomavirus can be seen as a single dense basophilic homogeneous intranuclear inclusion, pale nuclear inclusion creating a homogeneous space or clearing of the nuclei with a network of chromatin filaments (Fig. 14.6). These different morphologies are attributed to the different stages of infection. The importance of recognizing polyomavirus infection is the potential false positive diagnosis that these changes might induce, leading to unnecessary work-up. The hyperchromasia seen in the nuclei can mimic
carcinoma cells, thus leading to the term “decoy” cells. It is important to remember that the chromatin in urothelial carcinoma is not homogeneous and the filaments of chromatin seen in polyomavirus-infected cells are not seen in urothelial carcinoma.
carcinoma cells, thus leading to the term “decoy” cells. It is important to remember that the chromatin in urothelial carcinoma is not homogeneous and the filaments of chromatin seen in polyomavirus-infected cells are not seen in urothelial carcinoma.
Parasites
Several parasites can be seen in the urine specimen. They include Trichomonas vaginalis, Schistosoma haematobium, and Filariasis. Infection by the S. haematobium is commonly seen in parts of Africa, particularly along the Nile River. Abundant squamous cells and anucleated squames can be seen in the urine of infected patients due to the squamous metaplasia induced by the infection (7). A strong association of bladder infection by S. haematobium and squamous cell carcinoma has been reported.
Lithiasis
Lithiasis might represent a problem in the interpretation of urine specimens in the absence of proper clinical history. The presence of stones in the bladder can lead to increased cellularity in the urine specimen, presence of urothelial cell clusters with or without cellular atypia. The atypia can present in the form of hyperchromasia and prominent nucleoli. These changes might raise the possibility of a low-grade papillary lesion, which can show similar changes. Therefore, clinical history is paramount in the interpretation of these specimens.
Malakoplakia
Malakoplakia is a granulomatous inflammation that affects mainly middle-aged women (8). Histiocytes with eosinophilic cytoplasm containing basophilic inclusions with concentric laminations (Michaelis-Guttmann bodies) are present. These inclusions are periodic acid Schiff, calcium, and iron positive by histochemistry. Additionally, the urine specimen contains granular debris in the background and the urothelial cells display reactive changes (9).
Vesico-enteric fistula
Vesico-enteric fistulas can be a result of diverticulitis, colon cancer, inflammatory bowel disease, radiation therapy, or surgery (10). The urine specimens contain fecal material, such as vegetable cells and/or degenerated striated cells associated with amorphous debris and bacilli.
CYSTITIS GLANDULARIS/CYSTITIS CYSTICA
Cystitis glandularis and cystitis cystica are common changes seen in the bladder (11, 12). Cytologically, the specimens show the presence of few cohesive groups of bland columnar cells, which are occasionally associated with goblet cells. These groups are present in a clean background. The main differential diagnosis is adenocarcinoma, from which it can be
distinguished by the presence of a dirty background and nuclear atypia (coarse chromatin, pleomorphism, and nucleoli) in adenocarcinoma (13).
distinguished by the presence of a dirty background and nuclear atypia (coarse chromatin, pleomorphism, and nucleoli) in adenocarcinoma (13).
Squamous Metaplasia
Squamous metaplasia represents the replacement of the urothelium by stratified squamous epithelium (see Chapter 9), with the cytological findings as seen in other organs and is relatively common in premenopausal women (14). The presence of keratinized epithelium, particularly with atypia, warrants further evaluation.
Nephrogenic Adenoma
Cytologically, nephrogenic adenomas can present as cuboidal or columnarshaped cells with fine chromatin pattern and fine vacuolated cytoplasm (15, 16, 17, 18). The differential diagnoses include urothelial carcinoma, clear cell adenocarcinoma, and gastric adenocarcinoma. Reactive conditions also represent possible differential diagnosis. Clinical history and careful evaluation of the nucleoli are useful criteria in the correct diagnosis of nephrogenic adenoma. PAX-2 or PAX-8 may be useful in the follow-up of patients with recurrent nephrogenic adenoma (19, 20).
PRIMARY UROTHELIAL NEOPLASMS
Papilloma
Papillomas cannot be diagnosed by cytology reliably since these lesions are cytologically banal. Cytological specimens from patients with papilloma might yield an increased number of urothelial cells with minimal atypia and a small number of red blood cells (21). Inverted papillomas also cannot reliably be diagnosed in cytology specimens (22).
Papillary Urothelial Neoplasm of Low Malignant Potential
Papillary urothelial neoplasm of low malignant potential (PUNLMP) is defined as a urothelial tumor with papillary architecture but minimal cytologic or architectural disorder (23). These lesions are cytologically indistinguishable from low-grade urothelial carcinomas (23, 24, 25).
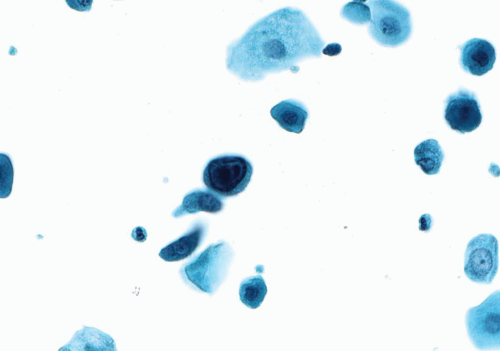
The urine specimens contain urothelial cells with homogeneous cytoplasm, slight cellular and nuclear enlargement, and eccentric nuclei. The nuclei have smooth nuclear contours and slightly granular cytoplasm and occasional nucleoli (Fig. 14.7). Mild hyperchromasia is noted (26, 27, 28, 29). These features are not uniformly present in all specimens and the diagnosis of PUNLMP based only on cytology is difficult at best and certainly not reproducible (30, 31, 32).
Papillary clusters have limited value in the diagnosis of PUNLMP except when a distinct fibrovascular core is noted (33). Papillary clusters in voided urine specimens can be due to reactive conditions such as inflammation and urolithiasis. Papillary-like clusters are common in cystoscopic
specimens due to the instrumentation (34). In summary, it is important to emphasize that an accurate diagnosis of PUNLMP relies on the cystoscopic and histologic findings rather than on the cytologic findings.
specimens due to the instrumentation (34). In summary, it is important to emphasize that an accurate diagnosis of PUNLMP relies on the cystoscopic and histologic findings rather than on the cytologic findings.
Low-Grade Papillary Urothelial Carcinoma
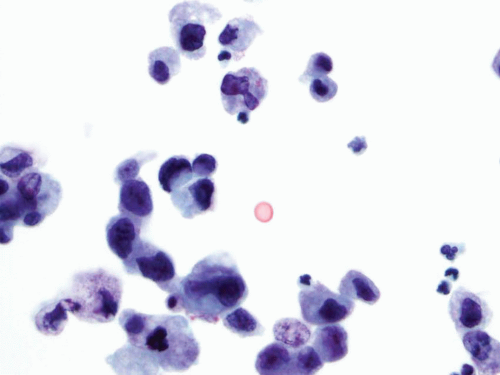
Given the high incidence, low-grade papillary urothelial carcinomas represent the major reason for the low sensitivity of urine cytology in the diagnosis of urothelial carcinoma. The urine specimens are heterogeneous with the presence of small and large clusters of urothelial cells. These urothelial cells have enlarged nuclei, with slightly higher N/C ratio and a small nucleolus (26, 27, 28). Some authors have suggested the presence of cells with a nucleolated globular body and variable slender cytoplasm as suggestive of low-grade papillary urothelial carcinoma (35, 36). Other authors have identified homogeneous cytoplasm, irregular nuclear membranes, and an increased N/C ratio as helpful criteria in the diagnosis of this lesion (30, 31) (Fig. 14.8). However, there is a lack of consensus in how reproducible these criteria are. The changes seen in low-grade papillary urothelial carcinoma can be hard to distinguish from morphologic changes due to lithiasis, inflammation, or even instrumentation. As previously mentioned, cystoscopic urine specimens from these conditions are usually cellular and contain clusters of urothelial cells that can include urothelial cells from the deeper layers of the urothelium. These cells are smaller, more hyperchromatic, and can form crowded clusters, making its distinction from low-grade urothelial carcinoma very difficult. On the other hand, the presence of cell clusters in voided urine from a patient without lithiasis or inflammation should raise the suspicion for a low-grade papillary
urothelial carcinoma, especially if the clusters have a fibrovascular core. The presence of markedly atypical cells in a cytology specimen in which the corresponding biopsy obtained at the same time shows a low-grade papillary urothelial carcinoma should raise concern and the patient should be worked up to rule out a co-existing high-grade urothelial carcinoma. The work up should include random biopsies of the bladder to exclude an associated CIS.
urothelial carcinoma, especially if the clusters have a fibrovascular core. The presence of markedly atypical cells in a cytology specimen in which the corresponding biopsy obtained at the same time shows a low-grade papillary urothelial carcinoma should raise concern and the patient should be worked up to rule out a co-existing high-grade urothelial carcinoma. The work up should include random biopsies of the bladder to exclude an associated CIS.
High-Grade Papillary Urothelial Carcinoma
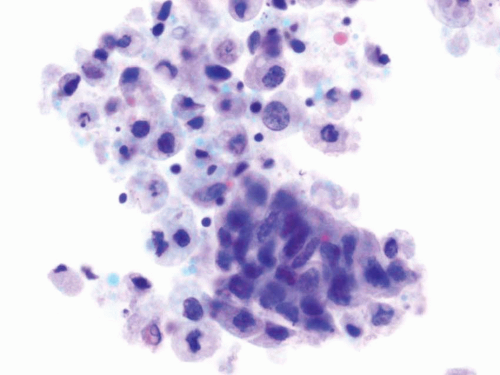
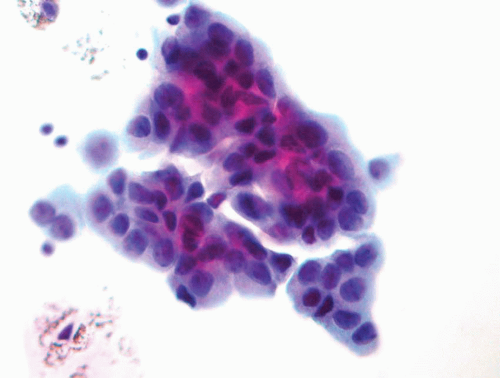
High-grade papillary urothelial carcinomas include some of the previously designated grade 2 and all of the grade 3 papillary urothelial carcinomas of the 1973 WHO classification. The cytology specimens, both in voided as well as cystoscopic samples, contain clusters and single markedly atypical cells with pleomorphic nuclei, coarse chromatin and, frequently, prominent nucleoli. The cytoplasm may be abundant and range from dense to vacuolated. The nuclear contours are irregular and the nuclei contain a coarse chromatin pattern which is irregularly distributed. Nucleoli are frequently present (Fig. 14.9). Invasion cannot be reproducibly diagnosed (28, 29), although some authors have associated the presence of cytophagocytosis with invasion (37).
Urothelial Carcinoma In-situ
Urine cytology represents a major tool in the diagnosis of disease since cystoscopy has a lower sensitivity due to several factors, including denudation of the urothelium. At cystoscopy, areas harboring CIS may be erythematous although other areas may be visually imperceptible (38, 39).
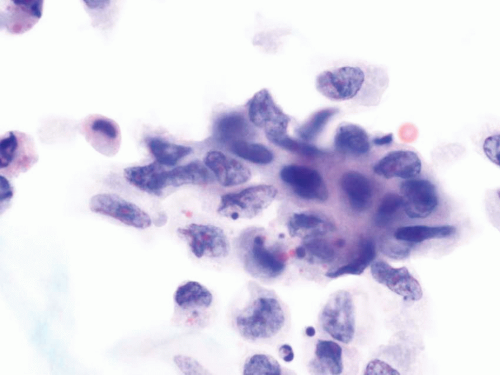
The voided specimens from cases with CIS contain a population of atypical cells of intermediate size which are present mostly as single cells, although clusters and large pleomorphic cells might be present. The cells are markedly atypical with hyperchromatic angulated nuclei, coarse chromatin pattern, irregular nuclear contours, and occasional prominent nucleoli. The N/C ratio is high and the cytoplasm is dense (Fig. 14.10). The differential
diagnosis includes cells infected by the polyoma virus (so called “decoy cells”), which have the characteristic nuclear inclusion. Degenerated cells can also mimic CIS due to the hyperchromasia and irregular nuclear contours. However, if the chromatin pattern cannot be evaluated, a diagnosis of malignancy should not be given. Treatment effect in patients receiving intravesical BCG or chemotherapy can also lead to a false positive diagnosis. These cells might be enlarged and contain hyperchromatic nuclei with prominent nucleoli. Clinical information of intravesical treatment is important and some studies suggest that UroVysion might help to achieve the correct diagnosis in these cases (40).
The voided specimens from cases with CIS contain a population of atypical cells of intermediate size which are present mostly as single cells, although clusters and large pleomorphic cells might be present. The cells are markedly atypical with hyperchromatic angulated nuclei, coarse chromatin pattern, irregular nuclear contours, and occasional prominent nucleoli. The N/C ratio is high and the cytoplasm is dense (Fig. 14.10). The differential
diagnosis includes cells infected by the polyoma virus (so called “decoy cells”), which have the characteristic nuclear inclusion. Degenerated cells can also mimic CIS due to the hyperchromasia and irregular nuclear contours. However, if the chromatin pattern cannot be evaluated, a diagnosis of malignancy should not be given. Treatment effect in patients receiving intravesical BCG or chemotherapy can also lead to a false positive diagnosis. These cells might be enlarged and contain hyperchromatic nuclei with prominent nucleoli. Clinical information of intravesical treatment is important and some studies suggest that UroVysion might help to achieve the correct diagnosis in these cases (40).
CORRELATION OF HISTOLOGIC GRADING WITH CYTOLOGY SPECIMENS
It has long been known that there is a proportional relationship between the diagnostic sensitivity of cytologic detection and the grade of urothelial carcinoma present. The sensitivity for high-grade urothelial carcinoma is high, while the cytologic diagnosis of low-grade urothelial neoplasms is associated with low sensitivity. The most sensitive method to detect low-grade urothelial carcinoma is cystoscopy and biopsy, which remains the gold standard. Papillary urothelial neoplasm of low malignant potential and low-grade carcinoma are very similar cytologically, making them indistinguishable by cytology (32). Additionally, the presence of papillary clusters in voided urine should also raise the differential diagnosis of lithiasis, strictures, and instrumentation effect. High-grade urothelial carcinomas are easier to diagnose due to its marked atypia associated with these tumors, but invasion cannot reliably be determined by cytology. Unfortunately, the current WHO/International Society of Urologic Pathologists (WHO/ISUP) bladder neoplasm classification system has led to a decrease in the accuracy of urine cytology in the diagnosis of high-grade lesions with no increase in the detection of low-grade urothelial carcinomas (24, 25). Based on the new WHO/ISUP classification, the Papanicolaou Society of Cytopathology has reported recommendations for the diagnosis of urinary cytology specimens as well as the suggested categories for each cytology diagnosis (Table 14.1) (3).
Invasive Urothelial Carcinoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree