Fig. 4.1
The semirigid ureteroscope (a = shown is the ACMI MR6 model) can be utilized to inspect the distal ureter, while the flexible ureteroscope (b = shown is the Karl Storz Flex Xc digital model) allows meticulous inspection of the entire ureter and intra-renal collecting system. Inset: the tips of each scope reveal the lighting element, lens, and working channel
With smaller fiberoptic bundles for imaging, the actively deflectable flexible ureteroscope was miniaturized to an average shaft diameter of slightly larger than 8 French. The flexible endoscope could now be passed into the ureter without a guidewire, thus minimizing the role of the semi-rigid ureteroscope [4, 5]. With improvement in distal active tip deflection, having a larger diameter with a maximum angle of the deflection of 270°, the endoscope could be passed through the intra-mural ureter under direct vision, and then proximally into the kidney [5]. A semirigid instrument was no longer required for all cases, employing a flexible instrument solely. Thus, as the fiberoptic endoscopes were miniaturized, the ability to evaluate an upper tract urothelial lesion in a particularly atraumatic fashion expanded (Fig. 4.1b). Complications decreased significantly, while the ability of the surgeon to map the collecting system broadened.
The strategy employed in evaluating an upper urinary tract filling defect or a suspicious lesion defined on contrast enhanced CT/MRI that is felt to be urothelial in origin, is based on first performing a careful diagnostic cystoscopy. Bladder barbotage urine specimens for cytopathology are obtained. Retrograde ureteropyelography is then performed with dilute contrast medium, helping to define areas that are particularly suspicious (Fig. 4.2). The flexible ureteroscope is then passed into the ureteral orifice under direct vision, if technically feasible. If the endoscope does not pass easily through the intramural tunnel, then a guidewire is placed through the instrument intubating the orifice as an obturator. If the endoscope continues to fail to pass thru the intramural segment, then a dilator is employed. Either a balloon or graduated dilator, no more than 12 French in diameter, is then employed to minimally dilate the intramural segment and thus decrease false positive findings on direct endoscopy.
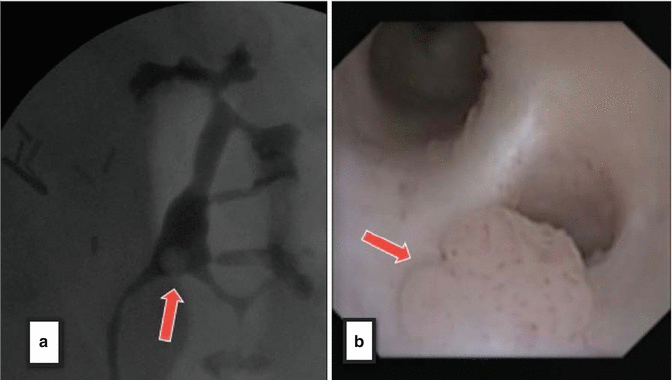

Fig. 4.2
(a) Left retrograde pyelogram depicting clear circular filling defect in renal pelvis (red arrow). (b) The digital flexible ureteroscope gives clear visual confirmation of tumor (red arrow) corresponding to filling defect
When mapping the upper urinary tract urothelium, the flexible endoscope is passed up the ureter under direct vision using sterile saline irrigant to clear the operative field. A barbotage specimen of saline is collected through the working channel of the endoscope during this evaluation for cytopathology. The intrarenal collecting system is carefully and meticulously inspected (Fig. 4.3). Suspicious lesions are sampled. For a papillary lesion (Fig. 4.5a), the application of a 2.4 French stainless steel based flat wire basket is an excellent accessory to obtain adequate tissue for histopathology. Tissue from the lesion is engaged in the basket, and a portion removed by extracting the endoscope and basket as a unit (Fig. 4.4). Specifically the basket is not pulled back through the working channel, rather the engaged tissue basket and endoscope are removed as a unit such that a sizable portion of tumor can be sent for histopathology. The endoscope is then replaced, and if the resultant hematuria associated with the biopsy is minimal, then additional biopsies and a second barbotage specimen for cytopathology is collected.
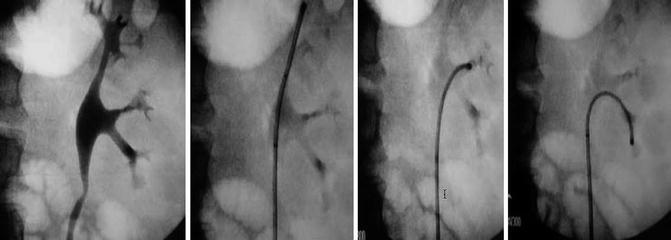
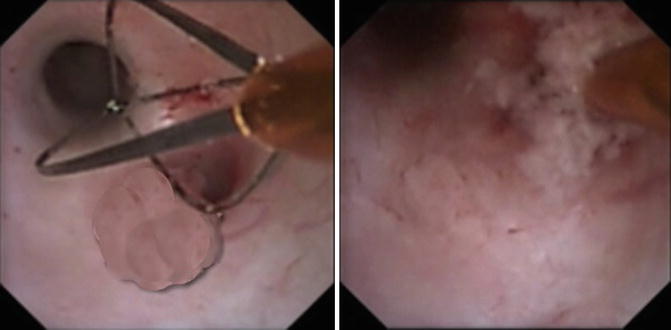
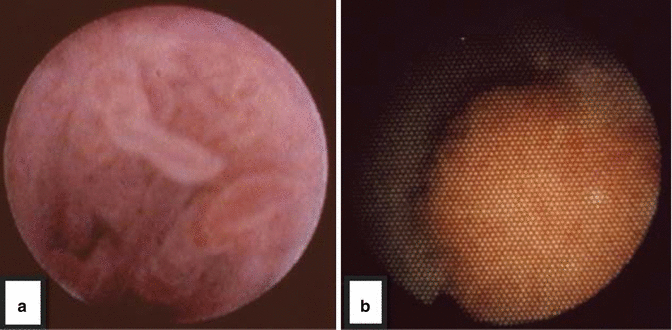

Fig. 4.3
The actively deflectable flexible ureteroscope allows for meticulous inspection/mapping of the entire intra-renal collecting system, thereby serving as the primary tool for diagnosis and treatment of upper tract pathology

Fig. 4.4
2.4 Fr Segura stainless steel basket is used to ensnare the tumor and obtain tissue for biopsy and pathological diagnosis

Fig. 4.5
(a) Endoscopic appearance of a papillary, low grade lesion- note shaggy appearance of frons with fibrovascular cores. (b) A high grade lesion, on the other hand, tends to be flat (sessile) and broad based
Flat, sessile, or small papillary lesions are difficult to biopsy ureteroscopically with a snare or flat wire basket (Fig. 4.5b). In those settings a small cup biopsy forceps is employed. The specimen, which is often a millimeter or smaller in size, is collected by extracting the forceps through the endoscope’s working channel. Multiple samplings with the cup forceps increase the sensitivity of this technique. The tiny specimens are released from the forceps by plunging the open cups into a conical tube under a few cc’s of saline. Multiple pieces are obtained of a suspicious flat area for example, with the specimen then prepared by the cytopathologist [6]. The conical tube is spun down to obtain a small tissue pellet, which is prepared employing cell block technique to improve the sensitivity of the biopsy. The supernatant is evaluated cytologically as well [6–8]. After ureteroscopic biopsy, another barbotage specimen is collected thru the endoscope’s working channel to help define any high-grade cells that may have been released with the act of mechanical biopsy, improving the sensitivity of the diagnostic procedure.
Commonly after performing an ureteroscopic biopsy, an internal ureteral stent will be placed to better ensure that the upper urinary tract remains drained. Frequently after a biopsy there will be associated hematuria. By employing a small electrocautery probe or laser energy through the ureteroscope, the base of the biopsy site can be coagulated for hemostasis, but frequently after the biopsy the involved segment is coated with a small portion of clot prohibiting this manuever. It is in that setting that a ureteral catheter is placed to help ensure drainage, minimizing clot colic, after this diagnostic procedure. Please see Chapter 1 for further discussion of diagnostic ureteroscopy.
4.3 Therapeutic Ureteropyeloscopy for Upper Urinary Tract Urothelial Malignancies: Technical Considerations
The treatment of upper urinary tract urothelial lesions varies based on the grade, structure, size, vascularity, and location of the encountered tumor [9]. Grade on presentation is the most significant prognosticator, and should direct to a large extent the crafted treatment algorithm [10–12]. Here again we mirror the experience in the lower urinary tract, where low grade lesions are often definitively treated, but recurrences are common and progression in grade with a recurrence will radically change therapy. Patients treated ureteroscopically in this setting must by counseled from the onset of therapy that lifelong endoscopic surveillance is an essential part of treatment. In addition post treatment surveillance includes periodic urine and serum studies, as well as additional imaging to define progression in stage [13].
Ureteroscopic therapy often begins with tissue sampling for histopathology, cytologic washings, and additional imaging studies for staging including standard imaging to assess for metastatic disease. After these baseline studies are completed, they are repeated at an interval over time to define recurrence and/or progression in grade and stage. Ureteroscopic treatment is employed in various settings: as definitive therapy in those with low grade lesions and favorable cytology, to palliate a symptomatic high grade lesion (e.g. addressing hematuria, un-obstructing the collecting system, etc.), or in those patients with high grade lesions who are not candidates for extirpative surgery secondary to co-morbidities or who refuse nephroureterectomy which may leave them with marginal renal function understanding that this therapy is most often palliative in this setting.
In the past, the majority of distal ureteral tumors were endoscopically treated with a 12 French uretero-resectoscope (Fig. 4.6). The addition and universal acceptance of small diameter fiberoptic semirigid endoscopes and actively deflectable, flexible ureteroscopes has led to abandonment of the larger and more traumatic instruments, rendering them almost obsolete. Current use of the uretero-resectoscope is, therefore, very limited and generally reserved for the largest distal ureteral tumors in a particularly dilated collecting system [14].
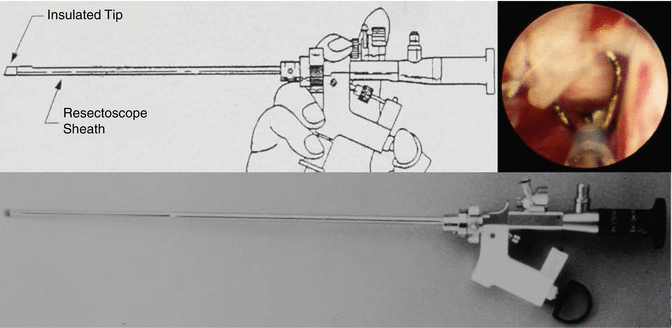

Fig. 4.6
The uretero-resectoscope being used to resect a distal ureteral papillary tumor (inset)
Papillary tumors that have bland papillae (Figs. 4.5a and 4.7) which are not particularly vascular can be treated with either electrocautery or holmium:YAG laser energy [15, 16]. The energy setting with regard to holmium laser energy is lower than those employed for lithotripsy. Low laser energies of 0.4–0.6 J, and frequency of pulsation that varies between 5 and 10 Hz, are common settings. Lower settings will ablate tumor while also coagulating the lesion, minimizing hematuria while maintaining a clear optical field (Fig. 4.8). Lesions that are papillary with sizable vascular cores and/or are particularly friable tend to bleed even with minimal manipulation and require a different treatment strategy. In that setting the base of the lesion is initially either coagulated with a 2 French Bugbee electrode or Nd:YAG laser energy is employed to de-vascularize the stalk, with subsequent application of holmium laser energy to remove (i.e. resect) the frons. The Nd:YAG laser settings are lower than what is employed for other solid tissue applications. Twenty to 30 W of power, employed for short periods though-out the visible tumor, will give optimal coagulation of the lesion, with holmium laser energy subsequently employed to clear (i.e. resect or ablate) the de-vascularized frons. It is important to note that higher settings of Nd:YAG laser energy applied for longer periods of time can be associated with significant tissue fibrosis, and can also create a sizeable acute soft tissue defect that can be associated with bleeding [17]. To minimize this, Nd:YAG laser wavelength should be used with lower power employed to a site for shorter periods. In addition, this wavelength should be used sparingly in the ureter, never circumferentially which has been associated with a high stricture rate.
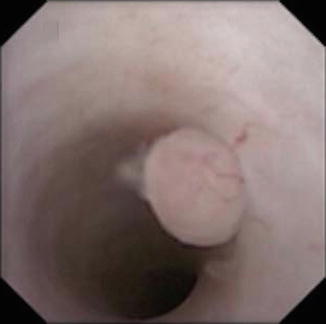
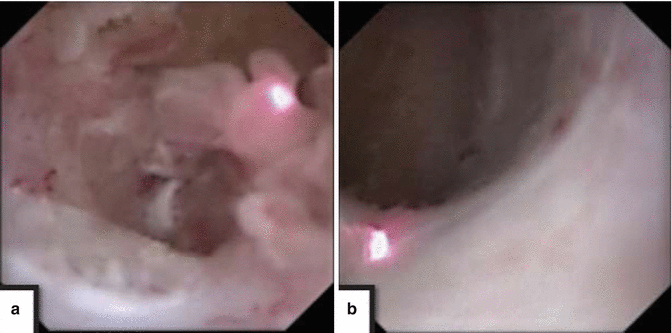

Fig. 4.7
Ureteroscopic view of a superficial, low grade urothelial tumor in the mid ureter

Fig. 4.8
Large volume papillary tumor filing a calyx (a) is carefully treated here with holmium:YAG laser energy resulting in clearance of tumor burden, down to flat mucosa (b)
Ureteroscopic treatment can obtain local control for particularly vascular high grade lesions but in general this treatment is commonly palliative [9, 10]. The most common presenting symptom is significant gross hematuria, where the surgeon is asked to stop the bleeding endoscopically. In that setting both Nd:YAG laser energy and electrocautery can be employed successfully. For lesions within the intra-renal collecting system, Nd:YAG laser energy can be employed around the tumor initially, from peripheral to central, in a non-contact mode, defocusing the light energy to create an area of thermal coagulation. Nd:Yag is avidly absorbed by hemoglobin, thus vascular lesions absorb this wavelength readily facilitating a deep coagulative effect [18, 19]. Electrocautery commonly produces a crust of coagulated tissue which may inhibit the efficiency of laser energy applied on the same tissue thereafter. In this setting of significant hematuria small aliquots of sterile water, limited to a small finite volume (i.e. less than 200 cc’s), will often clear the optical filed sufficiently so an energy source can be employed precisely to stop bleeding. It also important to remember that complete treatment commonly requires staged therapy, where coagulated tumor is allowed to slough, exposing the residua at the second sitting. Alternatively, holmium:YAG can be employed intermittently after Nd:Yag therapy to actively remove coagulated tumor exposing the lesions’ base.
Low grade lesions, specifically tumors that are biopsy proven low grade on histopathology and free of high grade cells on cytopathology from washings, are commonly treated ureteroscopically with the intent to cure [11–13]. Lesions that are large, either within in the intrarenal collecting system or ureter, and those that are circumferential in the ureter are often treated in stages. Treating a section of the ureteral wall, for example, and then allowing a period of healing with re-epithelialization before completing treatment minimizes the risk of stricture associated with thermal energy applied in this way. With ureteroscopic treatment of an extensive tumor burden, tumor is ablated at the first sitting, a ureteral stent is placed, and then patient returns after a short interval of healing (i.e. 1–2 weeks) for additional treatment after the devitalized tissue is allowed to slough. Staged therapy is also employed when extensive and/or multifocal tumor resection is required and when visualization is compromised due to bleeding during primary endoscopic resection [14].
For sizable intrarenal lesions that are particularly vascular, Nd:YAG laser energy can be employed to de-vascularize the lesion, with holmium laser energy employed immediately thereafter as a resecting wavelength. Applying these two laser wavelengths in sequence can efficiently remove a sizeable low grade lesion. A ureteral stent is regularly placed after the initial treatment, maintaining drainage while tumor sloughs, edema from thermal therapy resolves, especially between staged resections. Employing the staged methodology can facilitate clearance of even sizable lesions. Other relative indications for ureteral stenting post ureteroscopic treatment include a solitary renal unit, tumor multifocality, and tumor that is located in the intra-mural ureter. A period of stenting will better ensure drainage, and also during this period of healing, will allow the urothelium to regenerate in areas that were treated with one of the energy sources employed.
The endoscopist must be keenly aware of the limitations of the energy sources employed. Laser energy delivered in a pulsatile fashion will clear tumor similar to resection in the bladder, developing a smooth surface without visible tumor at the end of the treatment. Limits of laser energy though include the relatively stiff laser fibers that can inhibit the flexibility of the endoscope. Smaller diameter laser fibers are employed for lesions in the peripheral intrarenal collecting system, specifically in the lower pole. Lesions that are located in a peripheral calyx can be challenging to treat, particularly if tumor resides under the lip of a calyx or in a tortuous infundibulum. The holmium:YAG laser used at low energy can effectively coagulate tumor when placed in close proximity to a papillary projection. However, if a laser fiber cannot be focused directly on the tumor, i.e. under a calyceal lip to treat a rim of residual tumor, then a small 2 French electrocautery probe is often successful. The electrical energy is delivered circumferentially, as opposed to linearly from the tip of the laser fiber. The 2 French electrode can thus be placed adjacent to a tumor, and using either cutting or coagulation current the lesion is treated adjacent to the tip of the probe.
4.4 The Application of Adjuvant Topical Agents to Maximize Ureteroscopic Treatment of Upper Urinary Tract Urothelial Tumors
Various agents can be employed topically in the upper urinary tract to maximize therapy and minimize recurrence of an upper tract urothelial tumor, similar to their application in the lower urinary tract. Commonly this is employed immediately after ureteroscopic treatment, mirroring applications in the bladder immediately after transurethral resection [20]. Mitomycin C is the most frequently employed topical agent applied in the upper urinary tract [21–24].
From a technical perspective, the application of topical chemotherapeutic agents in the upper urinary tract requires an externally draining catheter. A single pigtail stent of relatively small diameter, most commonly 6 French, is employed which is secured to a bladder drainage (i.e. foley) catheter during treatment. This ureteral stent is positioned post ureteroscopic treatment into the upper urinary tract with the proximal pigtail precisely coiled in a predetermined segment of the intrarenal collecting system, preventing inadvertent instillation into tissue. In contrast, if an open ended catheter is employed the tip may inadvertently contact tissue, increasing the risk of absorption with significant systemic risk. For this reason as a routine the pigtail catheter’s position should be verified with contrast fluoroscopically before instillation of the topical agent.
A small aliquot of contrast (e.g. 2–10 cc’s depending on the volume of the intrarenal collecting system) is employed through the catheter with simultaneous real time fluoroscopic imaging to obtain a retrograde ureteropyelogram. If significant extravasation is noted at the end of the ureteroscopic procedure during this maneuver, then the application of the topical chemotherapy should be delayed and the catheter set to gravity until the upper urinary tract seals.
Mitomycin C solution for upper tract instillation most commonly is 20 mg in 100 cc of diluent. This is administered in a retrograde fashion through a single pigtail catheter over the course of approximately 1 h. Administration is carried out in the recovery room immediately after endoscopic treatment. This is a setting where patients can be carefully and continuously monitored during the treatment. The nursing staff is keenly aware of parameters that lead to stoppage of the instillation: specifically flank pain, fever, nausea and emesis, which all may reflect increased intrarenal pressure. Mitomycin solution is never actively pumped into the upper tract, but rather administered by gravity drip. If the intrarenal pressures are high reflecting obstruction then the gravity drip will stop, thus minimizing the risk of systemic absorption. Mitimycin C is also a topical irritant and can cause severe skin reactions. Patients are padded under their buttock and genitalia to collect any leakage from incontinence around the catheters. The mitomycin solution has a purple dye marker, and the nursing staff is clear that if they encounter this on the padding that the patient is immediately cleansed to minimize contact dermatitis and skin reaction.
The application of topical chemotherapy in the upper urinary tract is infrequently employed at the time of initial diagnostic ureteroscopy, and is most frequently employed in those patients with recurrence following a surveillance protocol after definitive therapy, or as part of staged ureteroscopic treatment for a sizeable or complex lesion. During surveillance, indications for topical therapy include either a sizeable or multifocal recurrence, or if the interval between recurrences is shortening. In those settings topical therapy is employed to minimize recurrence. Patients are carefully counseled on the risks associated with topical therapy, very similar to the counseling when this agent is employed in the bladder. See chapter X for further discussion of topical therapy.
4.5 Antegrade Endoscopic Treatment of Upper Urinary Tract Urothelial Malignancies
Before advanced retrograde flexible ureteropyeloscopic instrumentation became available, antegrade percutaneous endoscopic techniques were employed to treat upper urinary tract urothelial malignancies. The tenets of this form of endoscopic treatment were a staged placement of percutaneous access into the kidney, commonly allowing the tract to mature, followed by rigid endoscopic technique to treat papillary urothelial tumors. Limitations included the rigid instruments that were being employed which often could not access the entire intrarenal collecting system, the relatively high rate of significant hematuria requiring transfusion perioperatively, and the risk of tract seeding [25]. The addition of the flexible nephroscope, and subsequently the actively deflectable flexible ureteropyeloscope placed in an antegrade fashion down the ureter, broadened the indications for treatment and improved success rates in general [26].
Published series of patients with intrarenal urothelial malignancies treated in a percutaneous antegrade fashion have had relatively short follow-up with, in general, acceptable perioperative outcomes [27, 28]. The risk profile, however, associated with antegrade endoscopic treatment of an intrarenal urothelial tumor when compared to retrograde technique is significantly higher. Not only is there a higher risk of significant hematuria associated with this antegrade endoscopic resection, but the risk increases exponentially with repetitive procedures. Moreover, endoscopic surveillance protocols would logically be carried out using retrograde ureteroscopic technique henceforth. Thus the indications for antegrade endoscopic treatment of intrarenal urothelial malignancies are limited to those patients where retrograde access is not technically feasible (e.g. those with urinary diversions or a lower urinary tract reconstruction that prohibits retrograde access) or patients who for technical reasons the standard retrograde ureteropyeloscopic techniques are ineffective. One example is a patient with a large bulky papillary tumor where antegrade technique would more efficiently clear this significant volume of malignant tissue as compared to retrograde instrumentation requiring multiple sessions. Once again, this has to be weighed against the significantly higher risk of bleeding and potential renal loss from a percutaneous access in this setting. On the opposite side of the efficiency argument is the fact that relatively large lesions have been treated successfully ureteroscopically with significantly less morbidity [25, 29]. Retrograde treatment may require staged interventions to allow treated tumor to slough between applications of one of a variety of energy sources employed to coagulate visible tumor.
In summary, antegrade endoscopic treatment of an upper urinary tract urothelial malignancy has a limited role. The complications associated with this procedure are significantly greater than those associated with retrograde ureteroscopic therapy. The role of employing antegrade percutaneous tumor therapy is relegated to a select population of patients where retrograde access is not technically feasible, or in those patients where retrograde technique would be impractical based on clinical parameters defined at the time of presentation.
4.6 Retrograde Ureteropyeloscopic Treatment of Upper Urinary Tract Urothelial Malignancies Compared to Nephroureterectomy: Initial Success and Long-Term Outcomes
The published literature with regard to ureteroscopic treatment of upper urinary tract urothelial malignancies is limited, due to the relatively low incidence of this disease as compared to lower urinary tract tumors, the varied treatment protocols that were employed at various tertiary referral centers, and issues with regard to careful surveillance of the patient population defining long-term outcomes. We have carefully and fastidiously followed a patient population over 15 years with upper urinary tract urothelial lesions treated both ureteroscopically and with nephroureterectomy. This data has been published in sequence at different points of maturity, with the most recent 15-year follow-up presented in the British Journal of Urology in 2013 [29]. The presented database reflects a population of patients presenting with upper urinary tract urothelial tumors who were differentiated initially by grade at the time of diagnostic ureteroscopy and then placed in one of three treatment groups (Table 4.1).
Table 4.1
Patient demographics and findings at initial diagnosis
URS groups
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||
|---|---|---|



