Equipment list
Cystoscope (rigid and flexible)
Semirigid ureteroscope
Flexible ureteroscope
Nitinol guidewires (0.038″, 0.035″, Amplatz superstiff)
Hydrophilic guidewires—straight and angle-tipped (0.038″, 0.035″, 0.025″)
5 Fr open-ended ureteral catheter
5 Fr angiographic catheter (Berenstein)
Dual-lumen catheter or 8/10 Fr coaxial catheter
Ureteral balloon dilators—multiple sizes and lengths (4–10 cm, 12–18 Fr)
Biopsy devices (cup biopsy, brush biopsy)
Instruments for endoureterotomy (cold-knife, Holmium laser, electrocautery)
Ureteral stents (various sizes and lengths)
Endopyelotomy ureteral stent (various lengths)
Nephrostomy tubes (various sizes)
Methods to Access the Stricture
Retrograde Technique
Once the patient is anesthetized and placed in the dorsal lithotomy position, a cystoscope is placed in the bladder and the ureteral orifice is cannulated with a 5 Fr open-ended catheter for retrograde pyelography. The retrograde pyelogram will allow further definition of the ureteral stricture location, length, and nature (complete versus incomplete obstruction). A Nitinol guidewire is then passed up into the kidney under fluoroscopic guidance with the assistance of the open-ended catheter if needed. If the wire cannot be passed beyond the region of the stricture, a hydrophilic guidewire (angle-tip) can be attempted with the assistance of a 5-Fr open-ended ureteral catheter or angiographic catheter to pass beyond the stricture and up into the kidney. If a wire cannot be passed beyond the strictured area, an option is to complete the case and have a nephrostomy tube placed to relieve the obstructed moiety and plan a possible antegrade approach.
Urologists with more advanced ureteroscopic skills may proceed further in a retrograde manner if feasible and safe. The rigid or flexible ureteroscope can be passed over or adjacent to the working guidewire (or under direct vision) up to the level of the stricture and direct visual inspection of the strictured area can be performed (scope choice based on the stricture location). Via the ureteroscope, a guidewire may be able to be passed through a pinpoint stricture up to the kidney.
Antegrade Technique
An antegrade approach may be preferred with more proximally located strictures, cases in which a retrograde approach was unsuccessful and in cases of concurrent upper tract pathology such as intrarenal calculi. Upper or mid pole calyces are generally preferred to gain straight access to the ureter. Once the patient is anesthetized and placed in the prone position (or supine based on surgeon preference), percutaneous access is achieved if the patient does not already have a nephrostomy tube in place. Antegrade pyelography and fluoroscopy are used throughout the procedure to define anatomy and assist with accessing the region of the ureteral stricture. A guidewire is passed into the kidney under fluoroscopic guidance and if present the nephrostomy tube is removed. A 5-Fr angiographic catheter is passed over the wire and the guidewire is manipulated down the ureter. With the assistance of the angiographic catheter, the wire should able to be passed into the bladder. Similar techniques to the retrograde approach (as noted above) can be used to manipulate the guidewire to obtain access through the level of the stricture and down into the bladder. The wire can then be exchanged for an Amplatz superstiff wire. An 8/10 Fr coaxial catheter is then passed over the superstiff guidewire in order to place two wires down to the bladder (working and safety wires).
If a wire cannot be passed beyond the level of the stricture, a flexible ureteroscope can be passed antegrade over a working guidewire down to the level of the stricture and direct visual inspection of the strictured area can be performed similar to the retrograde approach. Through the ureteroscope, a guidewire may be able to be passed through a pinpoint stricture down to the bladder.
Combined Retrograde/Antegrade Approach
A combined approach can be used for complex ureteral strictures, particularly cases with complete obstruction, when simultaneous antegrade and retrograde access is necessary [41–46]. Several techniques have been described to treat these strictures. In one technique, the patient is placed in the supine position and contrast (with or without indigo carmine) is injected into the percutaneous nephrostomy tube. An ureteroscope is passed retrograde up to the level of the stricture, allowing identification of each end of the stricture under fluoroscopy and direct vision. While studying the stricture under fluoroscopy at various angles, an incision using Holmium laser towards the proximal segment is made and recanalization is verified when indigo carmine/contrast is noted. The incision is carried through to periureteral fat [43]. Hong and associates reported a 75% success rate with 23–67-month follow-up with this technique [43].
Another approach to complete ureteral strictures is the “cut to the light” technique [44]. After percutaneous access is achieved (as described above) the patient is placed in the dorsal lithotomy position and a flexible ureteroscope is passed antegrade to the proximal end of the stricture. Another ureteroscope is passed retrograde to the distal end of the stricture. The light of the scope on the side of intended endoureterotomy is turned off, the instruments are aligned, and the incision is made towards the other illuminated scope taking great care to not laser beyond the level of the stricture to prevent damage to the other ureteroscope. This technique can be helpful in short strictures with complete occlusion of the lumen.
Some authors have described similar techniques but instead of performing endoureterotomy the stiff end of a guidewire is pushed towards the light and the wire will then allow through-and-through access to allow different incision/dilation techniques to be employed [45–47]. Similar results have been reported in small series of complete ureteral obstruction [45–47].
Balloon Dilation and/or Endoureterotomy Technique for Ureteral Strictures
When performing balloon dilation without incision, the balloon is placed in the strictured segment and inflated under fluoroscopic guidance. Successful dilation is achieved once no “waist” is noted on fluoroscopy in the strictured segment. No uniform recommendations exist regarding balloon inflation duration or optimal number of inflations. Our technique typically determines the length of the balloon by the length of the stricture (most commonly using 4 cm dilators). We typically dilate to 15 Fr if the “waist” is effectively dilated with this size and leave the balloon inflated for approximately 1 min.
Several case reports and/or small series also report the use of balloon dilation with cryoplasty; a technique initially developed to treat peripheral vascular stenosis [48, 49]. This system employs a balloon catheter which is expanded and cooled to −10°C using nitrous oxide. The advantage of this technology is that it induces apoptosis in smooth muscle cells and fibroblasts that are generally involved in the scar response/restenosis. Additionally, theoretically by inducing apoptosis instead of necrosis there is a decrease in the amount of cytokines released and thus the inflammatory response, possibly limiting fibrosis/restenosis further [48–50]. Additional studies are needed to assess the safety and efficacy of this technology.
If an endoureterotomy incision is to be performed, it should be made away from periureteral vasculature (Fig. 5.1). Endoluminal ultrasound guidance has also been described to aid in identifying periureteral structures and directing the incision away from the vasculature [51]. The incision can be made using Holmium laser technology, cold-knife, or electrocautery. Our preferred technique uses Holmium laser at settings of 1 J at a rate of 10 Hz. We typically use 365-μm laser fibers when performing semirigid ureteroscopy and 200-μm fibers for flexible ureteroscopy. The advantages of Holmium laser technology for endoureterotomy include the small caliber of the laser, ability to achieve hemostasis with incision, and the depth of penetration is <0.5 mm limiting damage to neighboring blood vessels [52].
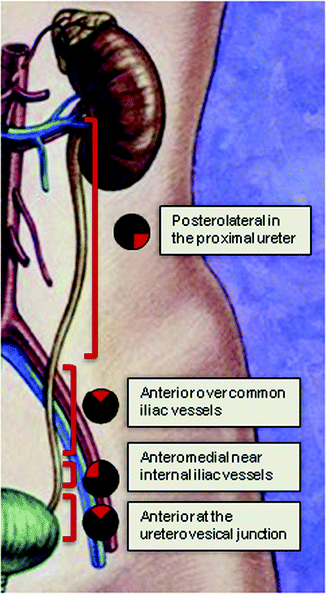

Fig. 5.1
The endoureterotomy incision should be made in the posterolateral portion of the proximal ureter, anteriorly over the iliac vessels, anteromedially over the internal iliac vessels, and then anteriorly over the ureterovesical junction
Traditionally, endoureterotomy with cold-knife incision uses a flexible, wire-mounted cold-knife that is inserted past the stricture segment under direct visualization or under fluoroscopy and pulled through the strictured segment with the use of a rigid ureteroscope (therefore used mostly for distal ureteral strictures) [53]. The lack of hemostatic capability with this technique can be a disadvantage since bleeding can easily obscure visualization. The invention of a blade-mounted guidewire has allowed the procedure to be performed antegrade, retrograde, and under direct vision [52].
Electrocautery incision can be performed with Greenwald Electrodes (2, 3, or 5 Fr) and endoscopic straight or right angle papillotomes (2–3 Fr) [52, 54–56]. These cutting instruments have the advantage of small size and ability to treat more strictures than the cold-knife as well as being able to provide hemostasis in addition to cutting through tissue. However, use of cautery can also lead to risk of scar tissue formation itself [52].
A hot-wire balloon cutting device (Acucise™—Applied Medical, Laguna Hills, CA) is another option for treatment of ureteral strictures performed endoscopically under fluoroscopic guidance. This device is a combination of monopolar electrocautery and balloon dilation placed cystoscopically under fluoroscopic guidance [57]. The system is inserted into the urinary system over a guide wire either retrograde or antegrade, the balloon is inflated with contrast, the stricture is assessed and incised using electrocautery until the waist of the narrowed segment is gone. The main disadvantage of this method is lack of direct visualization and potential for injury to adjacent structures, especially vasculature resulting in bleeding and possible need for further invasive procedure; therefore we do not recommend this technique based on its risks and potential for complications [58, 59].
Appropriate incision depth is achieved when periureteral adipose tissue is visualized; contrast extravasation can be used to confirm desired incision depth on fluoroscopy. The incision is extended into normal ureteral tissue generally 5 mm proximal and distal to the stricture and in cases of very proximal or very distal strictures, the incision extends into the renal pelvis or into the bladder or urinary diversion, respectively. Balloon dilation can be used prior to the incision to access the strictured area when too tight to pass a ureteroscope through and may allow further characterization of the narrowed segment. Balloon dilation following endoureterotomy can be both therapeutic and diagnostic allowing the surgeon to assess for obliteration of the strictured segment’s waist on fluoroscopy [55].
Ureteral Stent Selection
Indwelling ureteral stents placed following dilation and or endoureterotomy have several theoretical functions including scaffolding for healing, maintaining patency, and diversion of urine during the healing process. It is unclear whether larger stents are more effective in maintaining ureteral patency or more likely to cause fibrosis and recurrent strictures [60, 61]. Several prospective porcine models have found no difference in stent size (7 Fr versus 14 Fr) and recurrent stricture rates [62, 63]. One of these series did find a higher infection rate with larger stent size, and given that infection was associated with higher stricture rates, recommended using a smaller stent size [63].
Yu and colleagues found inserting two double J stents more effective than inserting one stent following balloon dilation of ureteral stricture, reporting success rates of 87.8% versus 62.7%, respectively [64]. Razdan and associates reported 80% patency rates on long-term follow-up in patients they considered high risk for failure (i.e., patients with longer strictures and history of radiation therapy) by placing two 6 or 6/8-Fr stents following endoscopic procedure for ureteral stricture [36].
The ideal ureteral stent dwelling duration is also unclear. Many leave indwelling stents for a 6-week period based on animal studies from the 1940s showing 90% muscular regeneration in a ureterotomy defect after 6 weeks [65]. Some porcine series reported increased infection rates with longer dwelling periods [63]. Another porcine model evaluating ideal duration of ureteral stenting found no difference on pathologic healing scores between ureteral stents left in for 1, 3, or 6 weeks and concluded that adequate duration of ureteral stenting following endoureterotomy may be as short as 1 week [66].
We place a double J ureteral stent (our preference is 8-Fr) or endopyelotomy stent (which can be inverted for distal ureteral strictures) for 4–6 weeks (which can be shortened or lengthened based on severity of stricture and operative course). If present, nephrostomy tubes should ideally be left open to drainage for 1–2 days following surgery to maximize drainage, then capped and/or removed after nephrostogram confirms patency. Fluoroscopic guidance should be used when removing a Cope-loop nephrostomy tube to ensure that the indwelling double J ureteral stent position is not affected.
When Endoscopic Treatment of Ureteral Strictures Fail
When balloon dilation and endoureterotomy fail, more invasive techniques may be required to achieve patency or placement of permanent or temporary ureteral stents/nephrostomy tubes with routine changes or nephrectomy. Reconstructive techniques include open, laparoscopic, or robot-assisted laparoscopic resection of the strictured ureteral segment with ureteroureterostomy, transureteroureterostomy, ureterocalicostomy, psoas hitch and/or Boari Flap, ileal transposition, or autotransplantation [67–71]. In cases where the patient’s condition precludes major reconstruction, but is symptomatic from the obstructed renal unit, nephrectomy may be the best option if the contralateral renal unit has adequate function.
Ureteroenteric Anastomotic Strictures
Etiology
Urinary diversion has become more commonplace over the last half century in the management of bladder cancer as well as some benign diseases of the urinary tract. One of the most common complications of this procedure is the development of anastomotic strictures. The workup of ureteroenteric strictures includes first ruling out new primary or recurrent malignancy with imaging, cytology, and/or biopsy. The most common benign causes of ureteroenteric strictures are ischemia, ureteral kinking, and inflammation following radiation therapy. The formation of anastomotic strictures may also be related to surgical technique, obesity (theoretically due to shorter ureters to mobilize, higher intraabdominal pressures leading to compromise of the anastomosis, and increased local secretion of cytokines from encroachment of adipose tissue resulting in impaired healing) [14, 19, 21]. The left ureter is at higher risk for ischemia and stricture secondary to more extensive dissection required for mobilization [19]. Non-refluxing ureteral anastomoses are also at higher risk of postoperative stricture [12, 72].
Technique for Endoscopic Management of Ureteroenteric Strictures
Similar preoperative preparation applies to the treatment of ureteroenteric strictures as ureteral strictures as previously described including treatment with culture-specific antibiotics prior to any intervention.
Retrograde Technique
Many urologists are discouraged to attempt retrograde access following urinary diversion given the additional challenge secondary to anatomic distortion even though an antegrade approach may have higher risk of bleeding, infection, and injury to adjacent organs such as the bowel and lungs and is often staged [73, 74]. Finding a ureteral orifice (UO), particularly in the setting of obstruction/stricture at the anastomosis, can be quite challenging in urinary diversions since normal tools to help identify the ureter (intravenous contrast or indigo carmine) will likely not work if the obstruction is high grade. Additionally, the UO can easily be obscured or difficult to locate within a diversion. In our experience, the level of challenge increases from ileal conduits to orthotopic neobladders to continent cutaneous urinary diversions.
Few studies describe the use of retrograde access alone when treating ureteroenteric strictures [6, 55, 75, 76]. Hyams and colleagues describe their experience with retrograde access in patients with urinary diversion in 21 renal units which included 9 cases for ureteroenteric strictures. This technique implements flexible cystoscopy for continent pouches and conduits or a rigid cystoscope for neobladders [76]. The urinary diversion is carefully inspected to identify the UOs based on the operative report’s description of the ureteral anastomotic location and what is typically expected with each type of urinary diversion. If the UOs are not readily identifiable, then a loopogram or cystography may be done to help delineate these structures in a patient with refluxing ureteroenteric anastomoses. If the patient has a neobladder with a Studer limb, indigo carmine may be given intravenously while the afferent limb is examined with flexible ureteroscopy [76]. Once the UO(s) of interest is identified, a guidewire is passed up into the kidney as previously described. If this approach fails, a 5-Fr open-ended angiographic catheter with a straight or angle-tip hydrophilic guidewire may be used. A working wire and safety wire should be placed using a dual-lumen catheter, given the challenge of achieving access in these patients. Generally flexible ureteroscopy is used, but rigid ureteroscopy is described for patients with a neobladder and direct access to the UO [76]. This series reported an overall 75% success rate for achieving retrograde access, with orthotopic neobladders having the highest success rate at 90%, followed by ileal conduits (73%) and Indiana pouches being the most difficult (33%) [76].
Antegrade Technique
The antegrade approach is similar to ureteral stricture disease described previously. In rare cases of ureterosigmoidostomy, there has been reports of successful antegrade balloon cautery endoureterotomy and balloon dilation alone for these ureteroenteric anastomotic strictures [77]. This series noted reflux of stool into the collecting system but had no cases of postoperative infections. They did not advocate preoperative bowel preparation, but did recommend a low residue diet while the stent was in place. In our limited experience with ureterosigmoidostomy anastomotic strictures we have had similar results, though employing a bowel preparation in cases where retrograde manipulation will also be performed.
Combined Retrograde/Antegrade Approach
In the case of combined approaches, positioning depends on type of urinary diversion. Patients with ileal conduits and cutaneous continent diversions should be placed in the supine position with access available to the flank region if a nephrostomy tube has been pre-placed. Patients with orthotopic neobladders may be placed in the modified lithotomy position with access to the flank region if a nephrostomy tube has already been placed [78].
One combined technique by Lovaco and colleagues describes intraluminal invagination to better visualize the stricture at the time of endoureterotomy [78]. This method uses an inflated balloon placed percutaneously and dilated in the ureteroenteric stricture while advancing into the urinary diversion to achieve intraluminal invagination. The most distal portion of the stricture is pushed into the urinary reservoir allowing for the stricture to be directly visualized within the intestinal segment and treated with a laser or electrocautery (such as a bugbee or Collin’s knife). This approach allows precise control of the incision under direct visualization and away from blood vessels and bowel [78]. This technique can also be extrapolated to be used in a retrograde manner if the ureteral orifice can be identified since this technique allows for a full thickness, full-length incision of the ureteroenteric stricture. At a median follow-up of 51 months (range 2–145) the success rate for endoureterotomy by intraluminal invagination was 80% (20 of 25 ureterointestinal anastomotic strictures) without complications.
Balloon Dilation and/or Endoureterotomy Technique for Ureteroenteric Strictures
Preoperative imaging should be carefully reviewed for nearby vasculature prior to endoureterotomy. Balloon dilation can be used alone or with concurrent endoureterotomy as previously described in ureteral stricture cases with endoureterotomy having higher success for longer strictures. When performing the endoureterotomy, the incision should extend 5 mm to 1 cm proximal to the stricture and extend into the reservoir or urinary conduit. When making the incision, some advocate incising circularly up to three to six times [53]. Some report making the incision posteriorly or laterally [5, 79, 80]. Selection and duration of ureteral stents is similar to ureteral strictures.
Kidney Transplant Ureteral Strictures
Kidneys are the most commonly transplanted organs with over 10,000 transplanted renal units per year in the USA. The most common urological complication following kidney transplantation is ureteral stricture occurring in 3–10% of cases with 80% of the strictures occurring at the ureterovesical junction (UVJ) [29, 81–83]. It is essential to relieve and correct these obstructions promptly in order to preserve graft function.
Endoscopic Techniques for Ureteral Strictures in Transplanted Kidneys
Following immediate decompression with percutaneous nephrostomy drainage and return of baseline renal function, a nephrostogram is performed for treatment planning of transplanted renal units with ureteral stenosis. Prognostic indicators for successful endourological management of these ureteral strictures include diagnosis of stricture a short time after transplantation and previous acute rejection [84]. An antegrade approach is frequently taken secondary to preoperative placement of nephrostomy and challenge of retrograde access, similar to antegrade technique previously described for ureteral strictures.
When taking a retrograde approach, as with ureteroenteric strictures, review of previous surgical reports can help identify the UO, which appears as an irregular region with stippled urothelium [85]. The transplantation technique also impacts the ease with which retrograde access is obtained, some reporting only 59% success when attempting access in patients who underwent the Lich-Gregoir technique versus 100% in those patients who underwent the Leadbetter-Politano method [86]. The rest of the procedure is similar to that previously described.
A combined antegrade/retrograde technique with endoscopic retrograde resection of a bladder window has also been described. This technique allows for direct retroperitoneal visualization and longitudinal incision of the distal ureter when the UO cannot be located on direct vision with or without the use of methylene blue [87]. This approach uses an angiographic catheter to create bladder wall motion to guide resection through the bladder wall with a resectoscope. Full thickness resection of the bladder wall allows entry into the retroperitoneal space and incision into the distal ureter longitudinally with the same resectoscope [87].
In cases where retrograde methods fail and there is a desire to pursue a minimally invasive approach without percutaneous puncture of the transplanted unit, laparoscopic pneumovesicular ureterotomy has also been described [88]. This technique implements laparoscopic surgery in the bladder cavity with pneumovesicum and incision into the stenosed ureter with placement of stent and an indwelling foley catheter for 3 days.
Once access is achieved, balloon dilation can be performed as previously described. When performing endoureterotomy, most describe making the incision anteriorly given that most strictures are located at the UVJ, or further assessment of the vasculature anatomy can be made with imaging and review of the prior operative report before performing endoureterotomy for more proximal strictures [89–91].
If endoscopic management fails other options include open, laparoscopic, or robotic-assisted procedures including ureteral reimplantation, segmental ureterectomy and psoas hitch and/or Boari flap, pyelovesicostomy, vesicocalicostomy, reimplantation with ileal ureter, or implantation of an artificial ureter [92–94]. Long-term ureteral stenting and nephrostomy tubes are also options but should only be considered in exceptional cases as the final solution in these immunosuppressed patients who are at even higher risk for infection [95]. For recurrent and recalcitrant ureteral strictures after kidney transplantation, placement of two double J stents has been shown to be successful [96].
Complications of Treating Ureteral Strictures
Complications from balloon dilation of strictures described in the literature include perirenal hemorrhage, urinary extravasation, hematuria, ureteroenteric fistula (successfully managed with ureteral stent only), fevers, UTIs/sepsis, balloon rupture, ureteral rupture, stent migration, and arteriovenous fistula (that necessitated transplant nephrectomy) [46, 97–102].
Complications from incision, especially when heat is involved, can be a little more complex. Complications with endoureterotomy include perforation, infection/sepsis, large volume intraperitoneal fluid extravasation, bleeding, injury to iliac vessels, urinoma, hyponatremia, ureteroenteric fistula, and stent migration [43, 44, 46, 55–57, 92, 103–109]. The most dangerous complication reported is large vessel injury that has been reported in cases of cold-knife incision and with a hot cutting balloon device [48, 54, 107].
Follow-Up after Ureteroscopic Treatment
When it is time for the stent to be removed, antibiotic prophylaxis should be started the day before stent removal and continue through the day after [110, 111]. There is no standard for postoperative monitoring for recurrence of stricture disease, but several series recommend an aggressive approach with renal scans, intravenous urograms, ultrasound, or CT imaging every 3 months for 2 years with decreasing frequency based on success of the procedure [53, 112]. Most failures occur within the first year; therefore close surveillance initially will prevent renal moiety obstruction and deterioration in renal function [41, 103, 105, 113, 114]. If failure occurs, depending on the condition of the patient and the stricture characteristics, decision will need to be made whether to continue with observation versus chronic stent or nephrostomy tube versus repeat endourologic approach versus ureteral reconstruction procedure or nephrectomy.
Factors Impacting Success Rates of Ureteroscopic Treatment
Renal Function
Decreased ipsilateral renal function is a known risk factor for failure of both endoscopic and open treatments of ureteral stricture disease secondary to low urine flow and decreased patency of stricture [103, 105, 113]. If ipsilateral split function is less than 15% on nuclear renography, strong consideration should be given to nephrectomy versus observation. Wolf and colleagues found that no patient with ipsilateral renal function less than 25% had successful endoureterotomy versus 80% success rate in strictures with ipsilateral function greater than 25% in a series of 47 ureteral strictures [41].
Stricture Length
Shorter ureteral stricture length strongly correlates with higher success rates in most series. Stricture length can usually be broken down into three categories: <1, 1–2, and >2 cm with decreasing ureteroscopic success as stricture length increases. Several series found ureteral stricture length to have statistically significant impact on the success or failure of endourological intervention [6, 41, 53, 98, 105, 113, 115]. One series of 50 patients mostly with benign ureteral stricture (two had ureteroenteric and five had malignant strictures) who underwent various endoscopic treatments found that all patients with strictures >2 cm failed endoscopic management while patients with stricture lengths ≤2 cm experienced 89% success rates [36].
Stricture Location
Multiple studies have reported higher failure rates for strictures located in the mid-ureter [56, 116]. The higher success rates in the proximal and distal ureters may be secondary to the ability to incise into a large cavity such as the renal pelvis or bladder and/or because of the better vascular supply in these segments of the ureter.
Stricture Etiology
Ureteral strictures due to ischemia (such as post-surgical, radiation related, cautery injury) develop significant fibrosis and can lead to lower success rates with endoscopic treatment. One series found that ischemic strictures undergoing laser endoureterotomy had statistically significant lower success rates of 64.7% versus 100% for ischemic versus non-ischemic, respectively [103]. Other series have corroborated these finding of higher failure rates in ischemic stricture disease undergoing ureteroscopic endoureterotomy [56, 105, 106].
Laterality
Unlike ureteral strictures, endoscopic interventions for left-sided ureteroenteric strictures have shown statistically significant lower success rates and trends toward higher failure rates compared to the right side [5, 41, 112, 117–119]. Most theorize that mobilization of the left ureter towards the right side compromises blood supply and results in an ischemic stricture [5].
Stricture Duration
Time from ureteral trauma to stricture formation also correlates with success of endoscopic treatment of ureteral stricture disease in some series [100, 105, 114, 120]. Theoretically, as strictures progress they tend to become more hypovascular and form denser scars with endoscopic treatments. Therefore, earlier intervention in more vascularized tissues should result in higher success rates. One large series reported no significant difference in success rates and stricture duration—however, this series’ definition of 24 months as early postoperative period for stricture formation was likely too long of a duration to show a difference as other series reporting more success with earlier identified strictures defined short duration as at least less than 1 year if not even shorter [41].
Stricture Steroid Injection
Reports of increased success rates in the treatment of other stricture diseases such as urethral and gastrointestinal by using concurrent steroids injections at the time of definitive management spurred some to try this method in ureteral stricture disease [121, 122]. Wolf and associates injected triamcinolone into the bed of 22 ureteral and ureteroenteric strictures and compared those to 46 strictures that did not undergo triamcinolone injection; they found statistically significant improved outcomes in those cases that received the steroids [41]. Meretyk and colleagues injected triamcinolone into the bed of a six ureteral strictures following endoureterotomy and had an 80% success rate in this cohort versus 50% in those not treated with steroid injection [56].
Evidence-Based Outcomes
Ureteral Stricture
The largest series evaluating the outcomes of endoureterotomy in patients with benign ureteral stricture disease reports 74–85% success rates with over 2-year follow-up (see Table 5.2) [36, 41, 123]. When looking at studies broken down by treatment type, there is some variation in success rates.
Table 5.2
Endoscopic management of benign ureteral strictures
Approach | Study | Number | Stricture lengtha (cm) | Mean follow-up (months) | Success rate (%) |
|---|---|---|---|---|---|
Balloon dilation | Yub [64] | 48/57 | 1.05/0.99 | 3–15 | 87.8/62.7c |
Anastasescu [97] | 12 | – | 87 | 75 | |
Lojanapiwat [106] | 18 | – | 9.7 | 60 | |
Myrend [125] | 40 | – | 7 | 85 | |
Hoe [100] | 22 | – | 12 | 73 | |
Beckman [120] | 28 | – | 1–40 | 79 | |
Johnson (1987) [114] | 24 | – | 6 | 62.5 | |
Laser incision | Lin [115] | 19 | 0.85 | 40.2 | 52.6 |
Gnessin [103] | 35 | – | 27 | 78.7 | |
Bach [126] | 18 | – | 12 | 61.2 | |
Hibi [127] | 17 | 2.07 | 60.5 | 76 | |
Lane [113] | 19 | 0.9 | 36 | 68.4 | |
Singal [128] | 18 | – | 10.8 | 76 | |
Cold-knife incision | Erdogru [105] | 13 | 1.3 | 9 | 54 |
Lojanapiwat [106] | 12 | – | 15.3 | 75 | |
Yamada [107] | 20 | 1.9 | 18 | 85 | |
Electrocautery incision | Conlin [46] | 2 | 3.75 | 46 | 50 |
Meretyk [56] | 13 | 3.0 | 20.4 | 62 | |
Balloon dilation cautery device | Seseke [57] | 15 | 1.3 | 15 | 53 |
Knowles [47] | 9 | – | 36 | 89 | |
Mixed studies | Corcorane [123] | 34 | 1.6 | 25.2 | 85 |
Razdanf [36]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




