Fig. 1.1
A retrograde with 30 % iodinated contrast mildly dilates and clearly outlines the ureter and the filling defect of the 3 cm neoplasm
Unless there is a finding on the contralateral ureteropyelogram, endoscopy is limited to the suspicious affected side. One of two “no touch techniques” is employed. This describes endoscopic inspection of the entire urothelium prior to any instrumentation. No guidewire which can traumatize the urothelium and the first and the earliest “no touch technique,” uses a small caliber rigid ureteroscope passed through the bladder and into the ureteral orifice under direct vision [83, 84]. The endoscope is advanced as far proximally as possible without excessive torque or angulation. Often in a female, the ureteroscope can be passed to the renal pelvis without difficulty, and the endoscope typically cannot be passed beyond the level of the mid-ureter at the iliac vessels in most patients. This initial passage of the radially dilating semi-rigid ureteroscope to inspect the distal and mid ureter mildly dilates the ureteral orifice and distal ureter and facilitates insertion of the flexible ureteroscope (FU). A guidewire is then passed through the rigid ureteroscope and left in place only to the most proximal level of the ureter inspected. The position of the wire is confirmed, both endoscopically and fluoroscopically. Strict attention should be taken to prevent inadvertent proximal migration of the wire. Dilation of the ureter should also be avoided to minimize trauma to the ureter from or the dilation itself. It is rarely necessary to dilate. Hudson et al. described that the ideal flexible ureteroscope outer diameter is 7.5 French (F) to minimize the need for ureteric dilation since <1 % of cases failed to pass using this size scope [85]. Flexible ureteroscopes from most manufacturers have increased in size to 8.3–8.5 F. Some digital ureteroscopes are even larger and may require dilation.
In the second “no touch technique”, the flexible ureteroscope is used alone without any initial rigid endoscopy. With the appropriate size ureter and flexible ureteroscope, it can be inserted directly into the ureteral orifice in a wireless, sheathless approach [86]. The flexible ureteroscope is passed through the urethra and bladder and then directly into the ureteral orifice. It is passed throughout the ureter to inspect the entire mucosa and then into the intrarenal collecting system.
As with the rigid technique, vision must be maintained through the passage of the flexible ureteroscope. Any small ureteral tumors are biopsied and treated as they are encountered using the techniques described below (Fig. 1.2). Such small lesions at 1–3 mm may be avulsed with scope advancement and be lost or possibly induce bleeding. Therefore, they are treated as they are encountered. If, in comparison, a large lesion is found, it may be helpful to advance the ureteroscope beyond the lesion into the more proximal ureteral segment to identify other tumors.
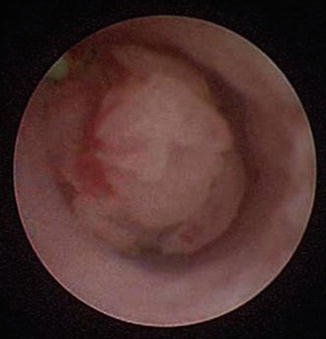

Fig. 1.2
Ureteral tumors visualized during initial ureteroscopy are sampled and treated to avoid damage and loss from instrumentation
With larger tumors such as those that fill the lumen and may extend 1–2 cm beyond the base (e.g. Fig. 1.1), it may be difficult to identify the ureteral lumen itself to get past the tumor. It may be useful to follow the contour of the ureteral wall or, in select cases, pass a hydromer coated guidewire beyond the tumor to gain proximal access. Although a “no touch technique” is preferred, a guidewire is used whenever it is needed to ensure access.
Next, the ureteroscope is advanced into the renal pelvis. An aspirate of urine is obtained and sent for cytology. This is completed by connecting a large (50–60 mL) Luer lock syringe directly to the irrigation port (Fig. 1.3). Avoid touching the pelvis and certainly avoid aspirating against the mucosa to avoid traumatic bleeding. The collecting system is then inspected systematically. First the entire pelvis is seen and then the intrarenal collecting system. Since severe deflection may be required to reach lower pole calices thus inducing bleeding, inspection should proceed from the renal pelvis to the upper, mid and finally the lower calices.
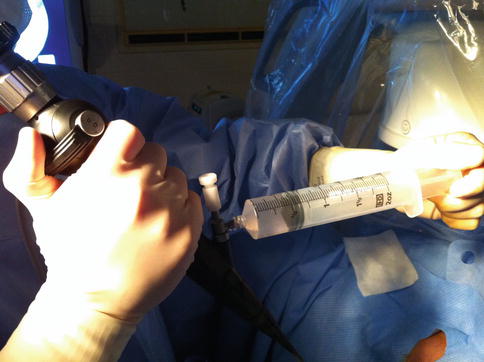

Fig. 1.3
Renal pelvic urine and irrigant is aspirated from the pelvis through the channel of the ureteroscope
Any tumors seen within the renal collecting system are biopsied and possibly treated at the end of the inspection. Various devices have been introduced to biopsy upper tract tumors, including baskets, forceps, brushes, snares and graspers. The most successful and most commonly used devices are the 3 French cup biopsy forceps and the stainless steel flat wire basket [87] (Fig. 1.4). The cup forceps are available in several designs of 1 mm diameter. These are well suited for sampling smaller papillary lesions, sessile or flat lesions. If the sample is entirely contained within the cup, the device can be withdrawn through the working channel. The device is then replaced through the ureteroscope and multiple samples are taken in a similar fashion to ensure a sufficient specimen for cytologic evaluation. If the tissue fragment extends beyond the cup, the entire unit consisting of FU, forceps, and biopsy specimen should be removed from the collecting system to preserve the largest specimen possible. The endoscope is then replaced and additional biopsies are obtained.


Fig. 1.4
A 1 mm biopsy forceps and a flat wire basket are commonly used for ureteroscopic biopsy [87]
Depending on the ureteroscope employed and cup forceps, the device may limit the deflection to only 90–100° [82]. This may prevent positioning to biopsy lower pole lesions.
The stainless steel flat wire basket is quite effective for more sizable, papillary, and friable tumors [83, 87]. The tumor is visualized and the basket extended and applied to the tumor itself. It is manipulated to bury the wires into the tumor and the basket is then closed snugly but not completely, which would cut the sample from the basket. It is used to avulse a piece of tissue. In this way, a large sample measuring up to several millimeters may be retrieved. These larger samples should not be withdrawn through the working channel since most of the tissue would be sheared off and lost. Instead, the entire unit consisting of FU, basket, and biopsy should always be withdrawn to maintain the largest tissue specimen possible (Fig. 1.5). The FU is reinserted and multiple biopsies are taken. With this flat wire technique, large tumors up to several cm in diameter can be mechanically debulked quite effectively. Kleinmann et al. compared the flat wire basket and cup biopsy forceps for the success rate of diagnosis and grade determination of UTUC. Diagnosis was successful in 63 and 94 % (P < 0.0001) and specific grade was determined in 80 and 93 % (P = 0.033) in the forceps and basket groups, respectively. On subgroup analysis of tumors larger than 10 mm in diameter, the flat wire basket was still shown to be superior in achieving pathologic diagnosis (P = 0.037) [87].
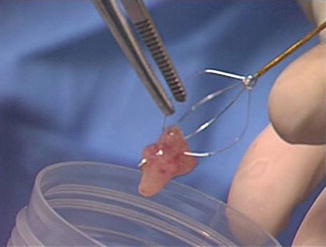

Fig. 1.5
The several mm tumor sample taken with the basket is transferred directly to the specimen cup containing saline. The sample can be lifted from the wires with forceps
Other devices are relatively ineffective and are rarely used in our practice. The nitinol round wire baskets, described as atraumatic, do not have enough grasping edge to capture tumor well. The brush is also very poor in retrieving tissue. When it is viewed directly endoscopically, the brush can be seen moving the tissue but not actually sampling it. It should be recalled, however, that cystoscopic placement of the brush to a filling defect improved sampling and diagnostic capability over voided urine alone [49]. Wire pronged graspers cause less loss of deflection with the flexible ureteroscope and may be used to sample tumors in the lower pole. However, the device cannot adequately sample or retrieve the tissue. More often, the prongs will tear off bits of the tissue which may be able to be retrieved by aspirating through the channel of the endoscope.
Recently, a cup forceps with a larger 2 mm cup has been introduced. Because of the size of the cup, the device must be back-loaded through the working channel of the FU. Subsequently, the flexible ureteroscope with the back-loaded forceps must be placed through a ureteral access sheath. If this technique is employed, we strongly recommend inspecting the ureter first with a “no touch technique” to evaluate for ureteral lesions prior to placing the access sheath. Next, it will be noted that the large cup forceps obscure the visual field. This device appears to have a more flexible shaft which allows better deflection. Although the sample achieved with the 2 mm forceps is greater than with the 1 mm, it is much smaller than that which can be retrieved with a flat wire basket.
1.8 Handling of Specimen
The tissue sample obtained with any of these techniques is transferred directly from the cup forceps or basket into a specimen container with normal saline (Fig. 1.5). It should not be placed first into a container in the operative field and then changed to the final specimen container. By minimizing the number of transfers, the chance of loss of specimen is minimized. If the delivery of the specimen to the Cytopathology laboratory is delayed (such as at night), the specimen is placed in a container with Saccomanno fixative. Preferably, fresh specimens are hand delivered to the laboratory where they are evaluated using the Cytospin smear technique. Importantly, all samples are processed as cytology specimens to avoid tissue loss during preparation [88]. Multiple samples are taken with either device to obtain sufficient tissue for pathologic analysis. In addition to the biopsy samples themselves, post-biopsy and post-laser aspirates are obtained for cytology (Also see Chap. 4).
1.9 Endoscopic Treatment
After biopsying the tumor adequately, it can be treated. It also should be noted that additional biopsy samples can be obtained, particularly if new or uninspected portions of the tumor are revealed during treatment. There are options among devices for endoscopic treatment. There are 2 and 3 F monopolar electrodes available which can be passed through the working channel of the flexible ureteroscope and powered by standard electrosurgical units available in almost any hospital. It may require special connecting cords which are generally available.
Lasers have assumed the dominant role in the endoscopic treatment of UTUC. The holmium (Ho) and neodymium (Nd):yttrium aluminum garnet (YAG) lasers have the largest role for treatment [82]. Some diode lasers have also been used. The Ho:YAG laser is a pulsed instrument with a wavelength of 2,100 nm. The energy from the Ho:YAG laser penetrates tissue only 0.5 mm so that its tissue effect is both superficial and visible endoscopically. It also must be positioned very close to the target tissue. This laser is primarily ablative but can also be used to coagulate tumors by defocusing the laser beam on the tissue by withdrawing it slightly from the target. Specific Ho:YAG lasers are available with a variable pulse duration. It is usually 350 μs but in those specific lasers can be increased to 700 μs. Its effect as seen as increasing coagulation. It also decreases the movement of stone during treatment.
The Nd:YAG laser has a continuous wave and a wavelength of 1,064 nm. Its penetration is deeper to approximately 5–10 mm. It is difficult to determine the depth of penetration so that it is best to follow its treatment in coagulation of tissue endoscopically. Both electrocautery and the Nd:YAG laser only coagulate tissue. The electrosurgical device must be in contact with the tissue while the Nd:YAG does not require contact, but in fact, can be a few mm from the tissue to be treated. Both should be used very carefully if at all in the ureter because of the risk of stricture formation. They should also be used very selectively within the kidney because of the risk of infundibular stenosis. Both are effective at coagulating bleeding sites. The Nd:YAG laser and less conveniently the electrocautery can be used in combination with the Ho:YAG laser. The tissue is first coagulated and then ablated with the Holmium.
The endoscopic treatment of tumor is discussed in greater depth in Chap. 5.
As noted above, all of the specimens which are at best small, are handled with cytopathologic techniques (Table 1.1). The specimens are delivered fresh in saline to the Cytopathology laboratory. The majority of the samples are prepared with the cytospin technique. If there is any macroscopically visible tissue in the sample, a cell block is also prepared. The latter can often demonstrate both of the architecture neoplasm and the individual cells to allow grading of urothelial carcinoma.
Table 1.1
Samples Obtained During Endoscopic Biopsy
1. Bladder urine |
2. Saline wash and aspirate from site of lesion |
3. Biopsy of the lesion (in saline) |
4. Post biopsy aspirate |
5. Post laser aspirate |
1.10 Cytospin Technique
The fresh specimen is prepared with Saccomanno fixative. If the specimen is grossly hematuric, a Cytorich red solution is added to lyse the red blood cells. The specimen is then centrifuged for 10 min at 1,200 RPM. Most of the supernatant is then discarded but 3–5 mL is placed into a special funnel apparatus, which contains a specimen slide, and again centrifuged for 10 min at 1,300 RPM. The cells are then adherent to the slide which is removed and air dried for 10 min. It is then placed in 95 % alcohol to remove the carbowax contained in the Saccomanno fixative and is subsequently inserted into the machine for Papanicolaou staining. The final step is to place the slide in Xylene bath for 2 min for clearing of the alcohol and then coverslipped.
1.11 Cell Block Preparation
A cell block should be prepared whenever there is any macroscopically visible tissue in the sample. This is done with the pellet obtained following the first centrifugation. The pellet is transferred to a fenestrated bag and wet with formalin. The bag is wrapped and placed in a cassette, which is immersed in formalin solution. The specimen is processed in several solutions, including two fixations in formalin, dehydration from 70 % alcohol through three absolute alcohol solutions, and clearing of alcohol in Xylene solution. It is then extracted from the bag and embedded in wax, sliced, and adhered to a glass slide. Lastly, the slides are stained with hematoxylin and eosin (H & E) and prepared as a histologic specimen. Architectural and cellular details of the lesion can often be demonstrated using the cell block preparation just as a histologic specimen.
Prior to using these techniques with a systematic approach, even the larger endoscopic biopsies tended to get lost during preparation. By handling all specimens in the Cytopathology laboratory, our diagnostic yield increased from 40 % to approximately 90 %.
1.12 Other Staining Techniques
Alternative fixatives and preservatives solution as well as stains are being evaluated in an attempt to improve the diagnostic yield in ureteroscopic biopsies. Bultitude et al. assessed whether Bouin’s fixative could improve the interpretation of ureteroscopic biopsies [89]. Bouin’s is a non-coagulate picrate solution which is routinely utilized to fix testicular biopsies because it preserves the nuclear detail. In this series, 28 pathological areas were studied in 18 patients. There were two groups of patients: Bouin’s group (7 patients, 10 pathological areas) versus 10 % Formalin group (11 patients, 18 pathological areas). The specimens were reviewed by two pathologists in a blinded fashion using an objective scoring system to evaluate five domains: specimen quality/size, specimen processing, architecture, cytoplasmic detail, and nuclear detail. No equivocal results were seen in the Bouin’s group but there were two equivocal biopsies in the formalin group. Nuclear detail was better preserved in the samples fixed in Bouin’s solution (p < 0.001).
The authors did note that Bouin’s solution is useful as either a fixative or staining solution, but it is not suitable as a preservative because of tissue shrinkage induced by the picric acid, tissues cannot be stored in Bouin’s solution for more than 48 h [89]. Also since the tissue is stained yellow, the solution makes specimen identification easier at the time of sampling and while embedding. The stain is removed with multiple ethanol washes. The authors concluded that Bouin’s fixative improved the assessment of ureteroscopic biopsies particularly with better preservation of nuclear details.
Special stains are also being studied to improve the pathologic analysis of ureteroscopic biopsies. Grading of UTUC has been challenging for interobserver agreement. Both mitotic figure counting and nuclear features are important in tumor grading but several factors such as apoptosis and artifact may interfere. Solomides et al. studied the use of a mitotic specific marker phospho-histone H3 (PHH3) as an adjunct to H & E stain for grading UTUC in cell blocks [90]. Formalin fixed paraffin embedded cell blocks from 61 upper tract urothelial carcinomas were stained with H & E followed by PHH3-antibody. Three pathologists graded tumors in a blinded fashion, first on H & E and then on H & E plus PHH3 stained slides. Gradings were compared between pathologists for each group. By adding the PHH3 stain to the H & E stain, the interobserver agreement in grading among the three pathologists improved dramatically and was statistically significant (average pairwise agreement = 80 %, overall kappa = 0.69). Therefore, PHH3 immunostain may improve grading of UTUC in small cell block samples.
1.13 Assisted Imaging Techniques
The visual diagnosis and sampling of UTUC remains imperfect. White light (WL) imaging is limited as the only modality in detecting the presence and extent of urothelial lesions, both in the bladder and in the upper urinary tract. Other optical diagnostics have been introduced in an attempt to extend these limitations [91]. Narrow band imaging (NBI) has been employed to enhance the visualization of more vascular structures. Similarly, the SPIES (Storz Professional Imaging Enhancement System) is another imaging enhancement modality being studied to improve the visualization of urothelial carcinoma. These techniques are discussed in greater detail in Chaps. 12 and 13.
The accuracy of ureteroscopic biopsies in relation to the final findings on pathology, particularly the tumor grade has been concerning. Several studies have demonstrated a biopsy concordance of up to 80–90 % between the endoscopic and final pathologic specimens [43, 44]. It appears that the accuracy can be enhanced by multiple samples dispersed throughout the tumor and associated cytology specimen. Preferably, the macroscopically visible samples should be used for cell block preparation. The techniques described in this chapter can be helpful in obtaining useful and accurate specimens. Most importantly, the samples should be delivered to the Cytopathology laboratory in saline as rapidly as possible to maintain their structure without cellular degradation. It is also most important to maintain contact and communication between the urologist and cytopathologist.
Conclusion
Although upper tract urothelial carcinoma is a rare malignancy, it is potentially lethal. Urinary markers may be helpful but improvement is needed before they can become a reliable modality for diagnosis. Ureteroscopic inspection and biopsy is an essential part of the evaluation of a filling defect or other suspicion of UTUC. The introduction of ureteroscopic treatment of upper tract tumor offers a nephron-sparing option in many patients. Accurate identification of the appropriate patient relies on adequate biopsy and careful specimen preparation. Extended imaging techniques may offer improved visualization and detection of urothelial lesions.
References
1.
2.
Flanigan RC. Urothelial tumors of the upper urinary tract. In: Campbell-Walsh urology. 9th ed. Philadelphia: Saunders Elsevier; 2007.
3.
4.
5.
6.
McLaughlin JK, Silverman DT, Hsing AW, Ross RK, Schoenberg JB, Yu MC, Stemhagen A, Lynch CF, Blot WJ, Fraumeni Jr JF. Cigarette smoking and cancers of the renal pelvis and ureter. Cancer Res. 1992;52(2):254–7.PubMed
7.
Ross RK, Paganini-Hill A, Landolph J, Gerkins V, Henderson BE. Analgesics, cigarette smoking, and other risk factors for cancer of the renal pelvis and ureter. Cancer Res. 1989;49(4):1045–8.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






