, Liqing Yao1 and Xinyu Qin2
(1)
Endoscopy Center, Zhongshan Hospital Fudan University, Shanghai, People’s Republic of China
(2)
General surgery, Zhongshan Hospital Fudan University, Shanghai, People’s Republic of China
Abstract
Tunnel endoscopy is a novel approach that was initially developed for the purpose of establishing an access for natural orifice transluminal endoscopic surgery (NOTES). This led to the idea of endoscopic procedures, using submucosal tunnel as an operating space. Esophageal achalasia treatment by Peroral endoscopic myotomy (POEM) marked the dawn of a new era therapeutic endoscopy [1–3]. Our group defined it as tunnel endoscopic surgery that allowed novel procedures utilizing a submucosal tunnel as an operating space. In 2010, we pioneered tunneling technique for treating upper gastrointestinal (GI) submucosal tumors (SMTs) originating from the muscularis propria layer and coined the acronym STER (submucosal tunneling endoscopic resection) [4]. Recently tunneling technique used to treat pyloric stenosis (peroral endoscopic submucosal pyloromyotomy) [5] and endoscopic submucosal tunnel dissection for large submucosal esophageal tumors [6].
Tunnel endoscopy is a novel approach that was initially developed for the purpose of establishing an access for natural orifice transluminal endoscopic surgery (NOTES). This led to the idea of endoscopic procedures, using submucosal tunnel as an operating space. Esophageal achalasia treatment by Peroral endoscopic myotomy (POEM) marked the dawn of a new era therapeutic endoscopy [1–3]. Our group defined it as tunnel endoscopic surgery that allowed novel procedures utilizing a submucosal tunnel as an operating space. In 2010, we pioneered tunneling technique for treating upper gastrointestinal (GI) submucosal tumors (SMTs) originating from the muscularis propria layer and coined the acronym STER (submucosal tunneling endoscopic resection) [4]. Recently tunneling technique used to treat pyloric stenosis (peroral endoscopic submucosal pyloromyotomy) [5] and endoscopic submucosal tunnel dissection for large submucosal esophageal tumors [6].
The development of tunnel endoscopic surgery is inspiring for both gastroenterologists and surgeons. The future of flexible endoscopy is not only operating in the natural orifice but also within and outside the gastrointestinal wall. If the lumen was historically the first and the peritoneal cavity the second, then the intramural space has come to represent the “third space.” GI endoscopy has finally entered the third space age [7].
6.1 The Rise of Tunnel Endoscopic Surgery
An interest in NOTES has grown since it was first reported in 2004. For safe access in NOTES two techniques were developed in porcine models. Sumiyama et al. reported “Submucosal endoscopy with mucosal flap safety valve” (SEMF) technique [8] and Moyer et al. described “self-approximating transluminal access technique” (STAT) [9]. The two independent groups shared the similar thoughts by creating a submucosal tunnel from the wall of natural orifice; however, the methodology of tunneling technique they used varied a little from each other. In SEMF technique, the authors created submucosal tunnel by high-pressure carbon dioxide (CO2) injection and balloon dissection, while in STAT, a fluid cushion of normal saline was made, and submucosal tunnel was created by the sharp and blunt dissection of a grasping forceps and the tip of the endoscope itself. A comparative study of four different transgastric access showed that an extended submucosal tunnel yielded the best leak resistance, which is superior to standard transgastric access methods and rival hand sewn interrupted stitches. However, neither balloon dissection nor forceps dissection could avoid the risk of bleeding when tunneling. The application of endoscopic submucosal dissection (ESD) technique in tunnel endoscopy optimized this transluminal access [10] (Figs. 6.1 and 6.2).
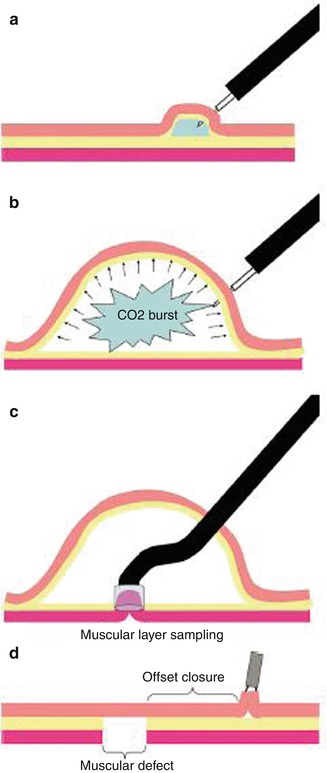
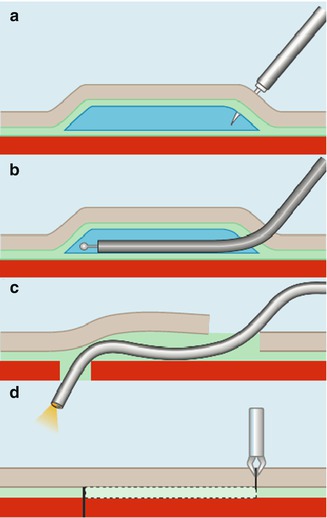

Fig. 6.1
Transesophageal mediastinoscopy by submucosal endoscopy with mucosal flap safety valve (SEMF) technique. (a) Saline-solution injection test to confirm needle-tip entry into the submucosa. (b) Gas submucosal dissection with high-pressure CO2. (c) Muscular-layer resection with cap-EMR technique inside of the submucosal space. (d) Offset closure of the muscular defect with overlying mucosal flap (Reproduced with permission from Sumiyama et al. [8])

Fig. 6.2
Submucosal tunneling using endoscopic submucosal dissection (ESD) for peritoneal access and closure in NOTES. (a) Submucosal injection with normal saline solution. (b) Creation of a longitudinal narrow submucosal tunnel using ESD. (c) Advancement of an endoscope into the peritoneal cavity. (d) Closure of the mucosal incision site using endoclips (Reproduced with permission from Yoshizumi et al. [10])
In 2007 Pasricha et al. carried out the first submucosal endoscopic esophageal myotomy in porcine model, a novel experimental approach for the treatment of achalasia [11]. In this procedure a submucosal tunnel created with a dilating balloon, the scope was then introduced into the submucosal space and the circular layer of muscle then incised using an electrocautery knife in a distal-to-proximal fashion, without complications. This animal experiment may be considered as the embryonic status of POEM (Fig. 6.3).
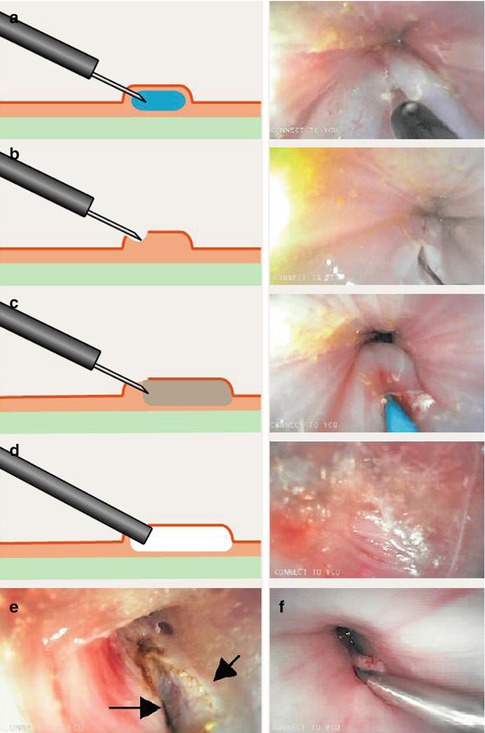

Fig. 6.3
Submucosal endoscopic esophageal myotomy. (a) Normal saline was first injected into the submucosa. (b) An incision was made using an electrocautery knife to provide entry for the balloon catheter. (c) Balloon dilation was performed to create the submucosal space. (d) The endoscope was then inserted into the submucosal space. (e) A myotomy of the circular muscle was then performed (the arrows point to the edges of the cut muscle). (f) The mucosal defect was finally closed with clips (Reproduced with permission from Pasricha et al. [11])
Inoue et al. reported the first series of 17 achalasia patients treated with POEM technique and paved the way of clinical application of tunnel endoscopic surgery [1]. Inspired by the success of POEM, other tunnel endoscopic procedures such as STER and endoscopic submucosal pyloromyotomy, were pioneered and generated great interest. Initial published experiences of tunnel endoscopic surgery is more than encouraging despite a relatively short follow-up.
6.2 Peroral Endoscopic Myotomy
Idiopathic achalasia is an uncommon esophageal motility disorder characterized by impaired relaxation of lower esophageal sphincter (LES). It has a prevalence of 1/10,000 and incidence of 1/200,000. Exact pathogenesis of the disease remains unclear, but underlying pathology is a lack of ganglion cell in myenteric plexus of esophageal wall leading to unopposed excitation of LES [12].
6.2.1 Clinical Symptoms
The predominant symptoms include dysphagia to liquid and solid food, regurgitation, retrosternal pains and weight loss. It can lead to respiratory complications (nocturnal cough and aspiration), malnutrition. Eckardt score is commonly used to grade disease severity (Table 6.1) [13]. This scoring system helps to evaluate improvement of symptoms after treatment (Table 6.2).
Table 6.1
Clinical scoring system for achalasia (Eckardt score)
Score | Symptom | |||
|---|---|---|---|---|
Weight loss (kg) | Dysphagia | Retrosternal pain | Regurgitation | |
0 | None | None | None | None |
1 | <5 | Occasional | Occasional | Occasional |
2 | 5–10 | Daily | Daily | Daily |
3 | >10 | Each meal | Each meal | Each meal |
Table 6.2
Clinical staging of achalasia
Stage | Eckardt scorea | Clinical implication |
|---|---|---|
0 | 0–1 | Remission |
I | 2–3 | Remission |
II | 4–6 | Treatment failure |
III | >6 | Treatment failure |
6.2.2 Diagnosis
The diagnosis of achalasia is confirmed on the basis of the absence of peristalsis and impaired relaxation of the LES.
Diagnosis is often suggested by Barium swallow by typical appearance of smooth tapering of the distal esophagus, known as the “bird’s beak”, with the dilation of the proximal esophagus and lack of peristalsis during fluoroscopy. Henderson et al. [14] described three grades in dilation of esophagus: Class I (mild), the diameter of esophagus <4 cm; Class II (moderate), the diameter of esophagus between in 4–6 cm; Class III (severe), the diameter of esophagus >6 cm, (dilated esophagus appears as sigmoid-type or megaesophagus). The timed barium swallow, is the modification of the barium swallow, and has the additional advantage of quantifying esophageal emptying time and can therefore be used as a simple and reproducible tool to assess outcomes after surgical or endoscopic treatment [15] (Fig. 6.4).
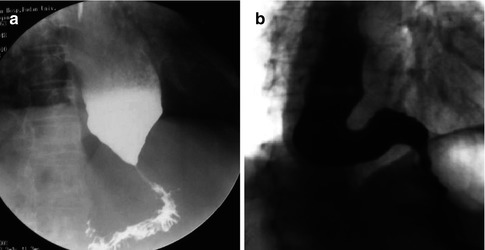

Fig. 6.4
Radiographic images of two patients with achalasia after a barium swallow. (a) Early achalasia. (b) Advanced achalasia with a sigmoid-type esophagus
Computed tomography (CT) scan, Magnetic resonance imaging (MRI) and transabdominal ultrasound are used not only to judge the degree of esophageal dilation but also to rule out neoplastic or infiltrative processes that can be the cause of pseudoachalasia (Fig. 6.5).
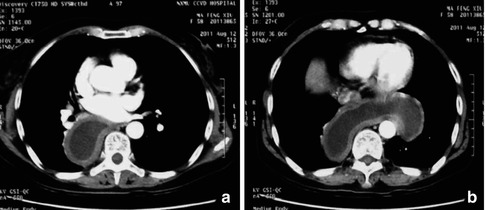

Fig. 6.5
CT images of the dilated esophagus
EGD is a valuable tool to detect the mucosal lesions and organic stricture result in pseudoachalasia. The features of achalasia in endoscopy: (1) retention of food or liquid, loss the normal mucosal color because of edema and thickening; (2) the proximal dilation of esophagus with torsion and deformity; (3) occasionally the lumen of esophagus above torsion bulges as a diverticulum; (4) different degrees of the cardia narrowing, the endoscope cannot go through it at times. Nevertheless, endoscopy frequently reveals normal findings with an early stage of achalasia (Fig. 6.6).
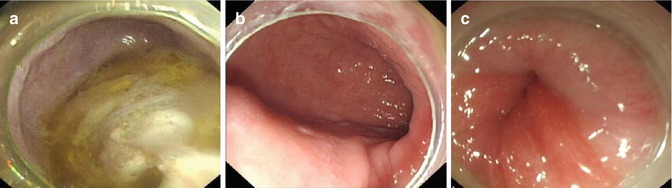

Fig. 6.6
Representative EGD images of achalasia. (a) Food remnants in the esophagus. (b) Dilated esophageal lumen. (c) Cardia stricture
Esophageal manometry remains the gold standard for the diagnosis of achalasia and typically shows three cardinal features of the disease: aperistalsis of the smooth muscle portion of the esophagus, impaired LES relaxation and, frequently, an increased LES pressure. Based on high-resolution manometry (HRM), Pandolfino et al. [16] described three distinct variants of achalasia: type I representing classic achalasia with minimal esophageal contractility and low intraesophageal pressure, type II representing aperistalsis and panesophageal pressure elevations, and type III representing lumen-obliterating esophageal spasm. Patients with type II achalasia often have the best overall treatment response, whereas patients with type III achalasia respond poorly to all types of therapy (Fig. 6.7).
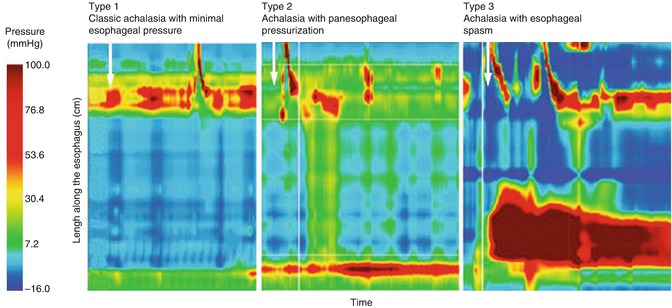

Fig. 6.7
High-resolution manometry (HRM) of the esophagus showing three different types of achalasia. White arrows indicate swallows (Reproduced with permission from Eckardt and Eckardt [17])
6.2.3 Treatments
To date, no treatment can restore esophageal function. Modern treatments focus on relaxation or mechanical disruption of the LES, aiming to relieve patients’ symptom of dysphagia, improving esophageal emptying, preventing the long-term development of mega-esophagus and restoring quality of life. The treatments mainly include pharmacologic, endoscopic and surgical treatment.
6.2.3.1 Treatments Before POEM
Pharmacological treatment including nitrates, calcium channel blockers, and nitric oxide donors (sildenafil) trialed but were less effective and poorly tolerated because of side effects.
Before POEM era endoscopic treatment options included: botulinum toxin (BT) injection, pneumatic dilation (PD), self-expanding metallic stents (SEMS).
BT is a potent inhibitor of acetylcholine release from nerve endings. Exocytosis of acetylcholine is inhibited and temporary paralysis of the innervated muscle occurs. BT counteracts the unopposed LES stimulation by cholinergic neurons, helping to restore the LES to a lower resting pressure. Clinical response to BT injection are short lived. Repeated injection may improve longer remission, but the response generally decreases with further injections, probably from antibody production to the foreign protein [18] (Fig. 6.8).
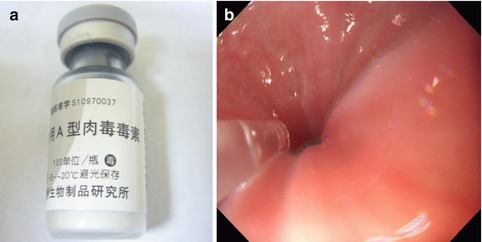

Fig. 6.8
Botulinum toxin (BT) injection for the treatment of achalasia
PD aims at disrupting the LES by forceful stretching using air-filled balloons. The first PD under fluoroscopy for achalasia was performed in 1971 by Vantrappen et al. [19]. With the development of endoscopy, investigators have performed PD using direct visualization. The standardized procedure with the Microinvasive Rigiflex balloon system (Boston Scientific Corp, Massachusetts, USA) which has three diameters (3.0, 3.5 and 4.0 cm) is: to position the balloon across gastro-esophageal junction. Balloon is then, gradually inflated with air to 7–15 psi in West (10–12 psi in Asian patients), for 15–60 s, until the waist is flattened [20, 21].
Long-term success is reported in 90 %, but usually serial dilations are required. Risk of perforation ranges up to 8 % [22] (Fig. 6.9).
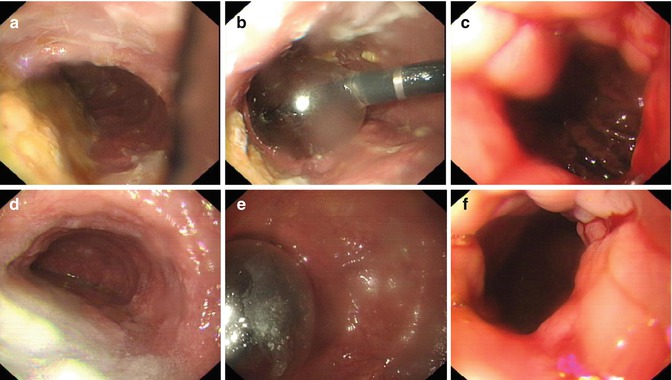

Fig. 6.9
Endoscopic PD for the treatment of achalasia
SEMS deployed placed across the esophagogastric junction (EGJ) under direct vision under conscious sedation. Temporary stents were removed after 3–7 days. De Palma and Catanzano [23] in reported pilot studies with SEMS (30 mm diameter) in 1998, outcome was poor with significant complications (Fig. 6.10).
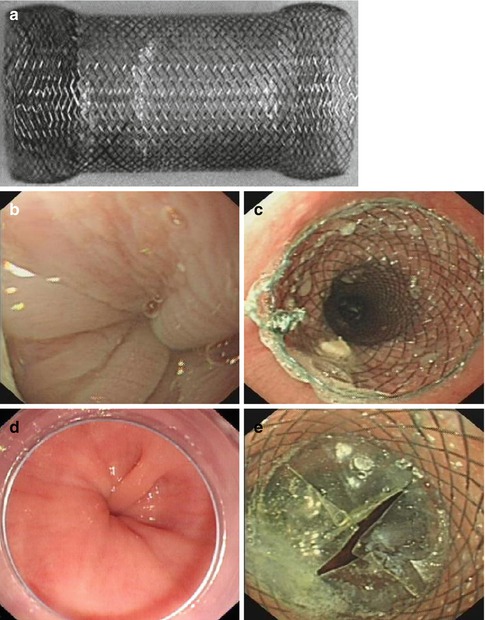

Fig. 6.10
Temporary self-expanding metallic stents (SEMS) placement for the treatment of achalasia. (a) A temporary SEMS. (b, d) The narrowing cardia. (c, e) Enlarged lumen of the GEJ after SEMS placement
First successful Surgical myotomy was performed by Ernest Heller et al. hence named Heller myotomy in 1913. Subsequently improvement in technique lead to the development of laparoscopic myotomy, thoracoscopic myotomy, ‘Needlescopic’ myotomy, lapro-endoscopic single site (LESS) myotomy and robotic telesurgery. A longitudinal incision is initiated on the gastric side approximately 2 cm distal to the EGJ and extended proximally 7 cm above the junction. In order to avoid long-term reflux complications, the procedure is usually combined with a partial anterior Dor fundoplication or posterior Toupet fundoplication. Surgical myotomy is usually reserved for endoscopic treatment failures. In some cases esophagectomy may be necessary because of the end stage sigmoid megaesophagus or development of complications such as esophageal squamous cell carcinoma and esophageal adenocarcinoma.
6.2.3.2 Peroral Endoscopic Myotomy (POEM)
Indications
All patients with achalasia can be treated by POEM. The obvious dilation of esophagus (sigmoid-type or megaesophagus), recurrence/persistence of symptoms after endoscopic treatment , Heller myotomy or initial POEM, may make subsequent endoscopic tunneling and myotomy more challenging, but in experienced hands usually does not prevent successful POEM.
Contraindications
Contraindications of POEM include severe cardiopulmonary disease or other serious disease leading to unacceptable surgical risk, pseudoachalasia, and failure in creating the submucosal tunnel because of severe fibrosis and adhesion, severe coagulopathy and esophageal or chest irradiation. Severe esophagitis and/or very large ulcer in the lower esophagus or EGJ should be considered as the relative contraindication, but mild or moderate esophagitis that does not cause stricture of the esophagus is not excluded (Figs. 6.11 and 6.12).
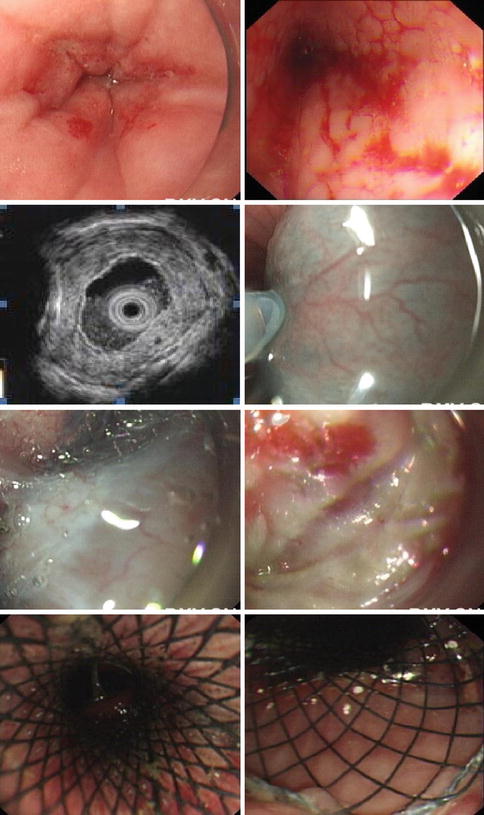
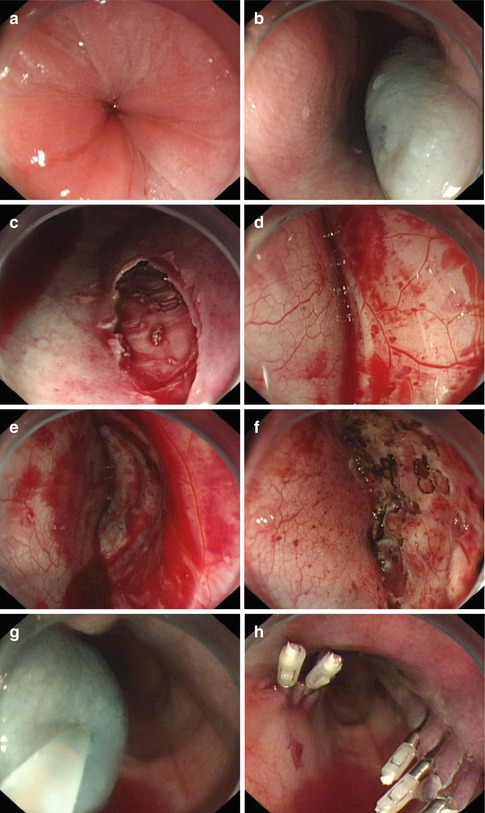

Fig. 6.11
A case of a 72-year old woman with 40 years’ history of achalasia. (a, b) Showed the narrowing of the lower esophagus (c) EUS showed thickened submucosal later with mixed echo. (d) Submucosal injection. (e, f) Submucosal tunneling failed due to severe fibrosis in the submucosal layer. (g, h) SEMS was then placed

Fig. 6.12
Failed case in a 12-year old child with 3 years’ history. (a) The narrowing cardia. (b) After mucosal injection. (c, d) No submucosal connective tissue between the muscularis mucosae and muscularis propria layer was found. (e, f) There was much oozing of blood on the surface of circular muscle layer. (g, h) Mucosal injection and incision were also attempted in opposite position but still failed
Operators
There is a substantial learning curve in performing POEM, and the best outcomes are likely to be achieved in medical centers with extensive experiences of endoscopic treatment. Experience is required for the operator and assisting team. The main operator should have wide experience of endoscopic resection (EMR, ESD etc), and had done at least 30 cases of esophageal ESD and able to deal with complications such as bleeding and perforation
Equipment
POEM is attempted with a single-channel gastroscope (GIF-H260, Olympus Medical Systems Co, Tokyo, Japan) and a hybrid knife (ERBE, Erbe Elektromedizin GmbH, Tübingen, Germany), triangle-tip knife (KD-640L; Olympus) or hook knife (KD-620LR, Olympus). A transparent cap (D-201-11802, Olympus) is attached to the tip of the gastroscope to facilitate entering and maintains endoscopic visualization in the submucosal space. The electrosurgical energy generator (VIO 200D, V1.7.10, Erbe Elektromedizin GmbH, Tuebingen, Germany) is used as radiofrequency surgical system that enables a spray-coagulation mode without contact tissue dissection. Other equipment include injection needle (NM-200L-0423, Olympus), Coag forceps (FD-410LR, Olympus), and clips (HX-610-90, HX-600-135, Olympus; ResolutionTM, Boston, MA). A slightly indigo carmine stained isotonic saline solution (3 ml in 250 ml) with diluted epinephrine (1: 250.000) is used for submucosal injection. Mucosal incision and muscular cutting are performed using ENDO CUT Q 3-1-4. Bleeding control is performed by the tips of knife using FORCED COAG E2, 60 W for vessels <1.5 mm or Coag forceps using FORCED COAG E2, 50 W for vessels >1.5 mm. CO2 gas is used for insufflations with a CO2 insufflator (UCR; Olympus) to reduce the gas-related complications. All equipments are sterilized by ethylene oxide gas (Fig. 6.13).


Fig. 6.13
Clinical settings of POEM. (a) Transparent distal cap attachment. (b) Triangle-tip knife. (c) High-frequency generator (VIO 200D, ERBE). (d) CO2 insufflator (UC; Olympus). (e) Hybrid knife (T type). (f) Coagulation forceps
Preparation Before POEM
Medical history, Eckardt score, barium swallow, manometry, and upper endoscopy are essential to make the correct diagnosis and clinical stage of disease, as well as to assess the complexity of procedure. Pulmonary function test are carried out in patients who have the history of severe pulmonary disease. Procedure with its benefits and potential complications are discussed with the patient and written informed consent obtained. All patients are given liquid diet for 2 days before surgery. A gastric tube is inserted to drain the contents of the esophagus when necessary. A preoperative endoscopy is performed to clean out food remnants from the esophagus to avoid aspiration during induction of anesthesia. Patient kept nil by mot for 8 h.
POEM Procedure
POEM is performed under general anesthesia with positive pressure ventilation. Patient is placed in the supine position or the left lateral position, administered intravenous antibiotic prophylaxis (the first or second generation cephalosporin) 30 min before the procedure (Fig. 6.14).
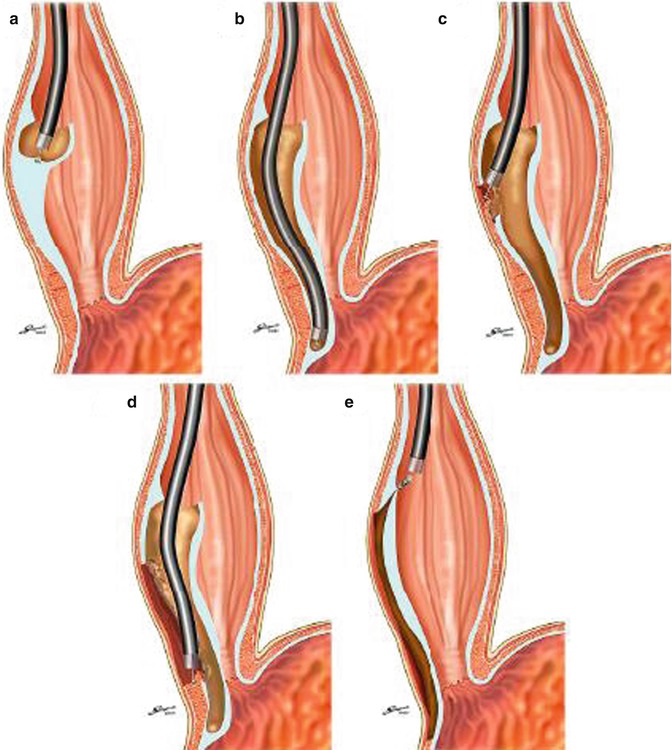

Fig. 6.14
Schematic depiction of the POEM technique. (a) Submucosal injection and mucosal incision toward submucosal space. (b) Creation of submucosal tunnel. (c) Myotomy started at inside submucosal tunnel. (d) Completion of myotomy beyond EGJ. (e) Closure of mucosal entry (Reproduced with permission from Inoue et al. [24])
Step 1: Mucosal incision. A submucosal injection (saline, indigocarmine and diluted epinephrine) is used at 5–6 o’clock to expand submucosal space 10–15 cm proximal to the EGJ, followed by 1.5–2 cm long mucosal incision (Fig. 6.15).
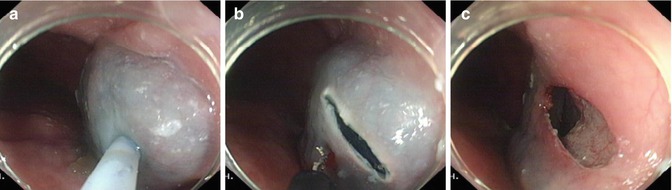

Fig. 6.15
A 2 cm longitudinal mucosal incision was made to create a mucosal entry
Step 2: Submucosal tunnel. After the mucosal incision endoscope is inserted into the submucosal space. Submucosal tunnel is created with sequential submucosal injection and submucosal dissection using EST technique. The tunnel is extended beyond the EGJ, 3–4 cm into submucosa of the cardia. The width of the tunnel is about approximately one-third of the circumference of the esophagus (Fig. 6.16).
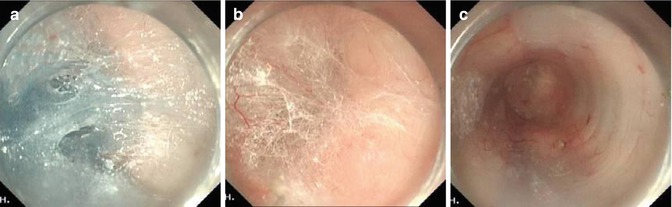

Fig. 6.16
With ESD technique, a submucosal tunnel was created downwards, passing EGJ, and about 3–4 cm into the proximal stomach
Submucosal dissection is carried out close to the muscular layer with sequential saline injection to reduce the possibility of mucosal injury. A mucosal injury or perforation within the tunnel may lead to mediastinal sepsis because the mucosa is the only barrier between the esophageal lumen and the mediastinum after full-thickness myotomy (Fig. 6.17).
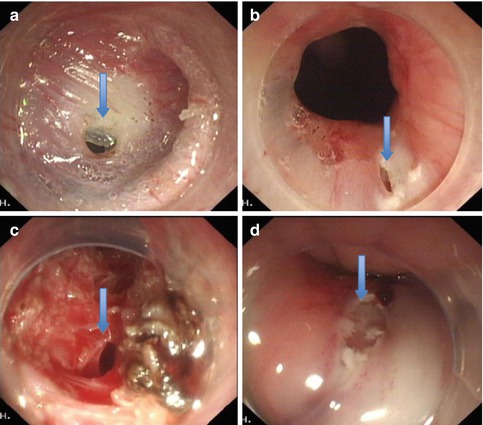

Fig. 6.17
The fibrosis at the EGJ is common for pre-treatment patients. Sufficient submucosal injection is important to prevent mucosal injury (arrows show the mucosal rupture)
During submucosal tunneling, the location of the distal margin of the tunnel can be identified according to the color change of mucosa by withdrawing the endoscope and using a retro-view from within the gastric lumen. There are five tips to identify the EGJ. The first indicator of the location of the EGJ is the insertion depth of the endoscope (centimeter marks on endoscope). The second indicator is an obvious increase in resistance when the endoscope is at the level of the EGJ, followed by an immediate easing when the endoscope passes through the EGJ and enters the gastric submucosal space. The third indicator is the palisade vessels on the underside of mucosal layer at the EGJ. The palisade vessels are located at the distal end of the esophagus. The fourth indicator is the degree of vascularity in the submucosal layer. Lastly blue discoloration of cardia mucosa can be seen on retroflexion in the stomach (blue dye used in submucosal injection) (Fig. 6.18).
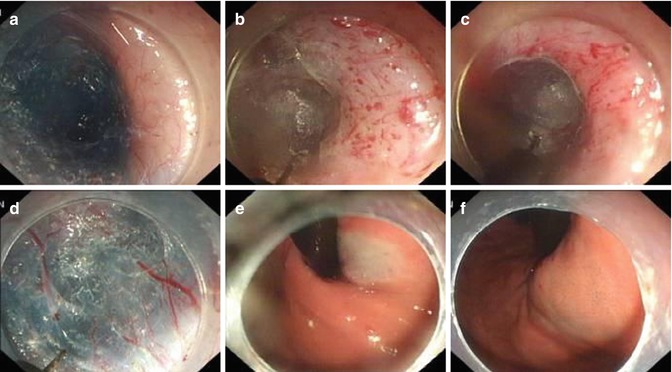

Fig. 6.18
Identification of the EGJ and distal margin of the tunnel via typical vessels and color changes respectively. (a) Few vessels exist in the esophageal submucosal layer. (b–d) Palisade vessels within the gastric submucosal layer. (e, f) Mucosal color change at EGJ
Step 3: Endoscopic myotomy. Endoscope is withdrawn to approximately 2 cm below the mucosal entry point, the circular muscular layer is then selectively dissected, thus offsetting mucosal entry incision and the muscle defect, allowing safe mucosal seal afterwards. Myotomy is extended 2–3 cm below the EGJ into the cardia. We routinely divide muscle fibers over a minimum length of 6–8 cm in the esophagus and at least 2 cm onto the cardia according to the surgical standards of Heller myotomy. Substantial reduction of LES tone is confirmed by opening the EGJ with gentle insufflation through the endoscope and easy passage of endoscope into stomach. In patients with chest pain caused by the abnormal contractions of hypertrophied muscle within the esophageal body (often considered as type III achalasia on HRM), a long myotomy is made to include all abnormal contractions (endoscopically visible and measured with manometry). During the procedure, minor bleeding is often treated by forced coagulation using the tip of the knife. Pulsating bleeding point from larger vessels is generally grasped and coagulated with a hemostatic forceps using the soft coagulation mode. When large vessels are visible during operation, they are precoagulated using the hemostatic forceps in soft coagulation mode (Figs. 6.19 and 6.20).
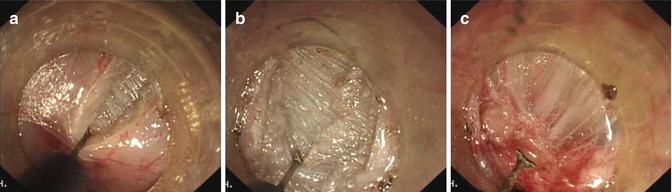
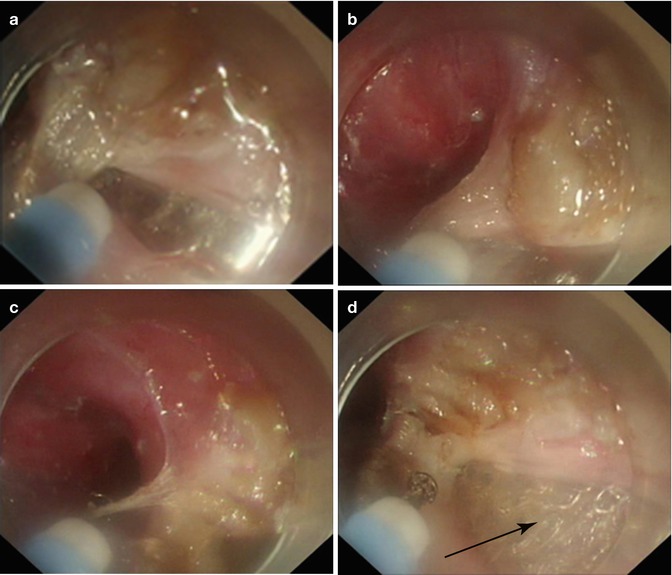

Fig. 6.19
The thickness of the inner circular muscle varies among achalasia patients. (a–c) Dissection of the circular muscle bundle was performed layer-by-layer

Fig. 6.20
POEM with a full-thickness myotomy. (a–d) Sequence of a full-thickness myotomy being performed at the right lateroposterior wall. Note after clinging to the longitudinal muscle fibers and lifting them up toward the esophageal lumen, the full-thickness muscle bundles being sectioned. The arrow indicates the external coat of the distal esophagus
Step 4: Closure of mucosal entry. After careful hemostasis within submucosal tunnel, the mucosal incision site is closed with several hemostatic clips (Fig. 6.21).
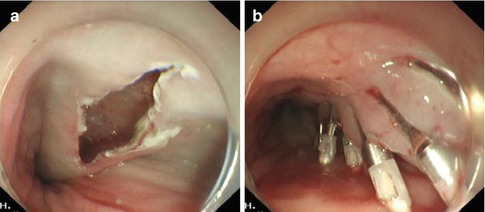

Fig. 6.21
Closure of mucosal entry: the mucosal incision is closed using hemostatic clips
Current Controversies
Length of Myotomy
Initial myotomies by Inoue were 3–5 cm, but after safety of this procedure confirmed, mean myotomy length has been extended to 8–10 cm (6–8 cm in esophagus and 2 cm into cardia). We believe that in future myotomy length in patients will be tailor made to manometric findings.
Myotomy Orientation in Relation to LES
LES consist of thin circular muscle fiber component on lesser curve at 2-o’ clock and an oblique sling of muscle fibers component at about 11 and 5-o’ clock. These oblique fibers by maintaining angle of his offer a significant anti-reflux barrier. Myotomy at our center is orientated at 2-o’clock position, cutting the circular fibers and sparing the oblique fiber hence preserving anti-reflux mechanism. Myotomy orientation on the other hand in laparoscopic heller myotomy (LHM) is at 11-o’clock, resulting partial transection of anterior sling fibers. We propose potential tailoring myotomy orientation depending on LES pressure and tightness.
Circular Muscle Layer Myotomy Versus Complete Myotomy
Although a circular muscle myotomy preserving the longitudinal outer esophageal muscular layer is often recommended (which is different from the usual full-thickness myotomy performed surgically) in order to avoid entering the pleural space to reduce morbidity. It is often difficult to preserve the longitudinal muscle as they are extremely thin and frequently split unintentionally during POEM even with minor electrocautery damage or mechanical trauma from endoscope maneuvering in the tunnel, even with air insufflation alone. In fact, a clear separation of circular and longitudinal muscular layers, is not easy to make at the EGJ and stomach. Moreover, completeness of myotomy is a prerequisite for sufficient and long-term reduction of LES pressure and is the basis for the excellent result. Given the fact that an incomplete myotomy with possible fibrotic healing can result in postoperative recurrence, a full-thickness myotomy theoretically induce long-term symptom remission. For these considerations, POEM with a full-thickness myotomy including the internal circular and longitudinal muscular layer has been performed in about half of the achalasia patients in our center. On the basis of more than 600 cases’ experiences in our center, short-term symptom relief and manometry outcomes were comparable between patients undergoing full-thickness and circular muscle myotomy. Full-thickness myotomy significantly reduced the procedure time but did not increase the procedure-related adverse events or clinical reflux complications. Long-term data on remission will confirm our recommendation. We prefer and recommend full-thickness myotomy in clinically symptomatic patients especially form 5 cm above the EGJ to 2–3 cm below [25].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








