Fig. 20.1
Histology of a tubercular lesion with caseation, multinucleated giant cells, macrophages, and lymphocytes
Pathogenesis
An active extrarectal tuberculous lesion or a history of previous infection can usually be elicited in these patients. Perianal tuberculosis appears in several forms. The commonest are the perirectal abscess, usually with secondary infection, fistula in ano, and a soft, indolent perianal ulcer. If an abscess is formed, it may appear as the typical perianal or ischiorectal variety with secondary infection, or it may remain for a time as a non-tender swelling with induration of the subcutaneous tissues near the mucous margin. Eventually this becomes secondarily infected to soften and break and then to persist as an indolent swelling with profuse thin purulent or watery discharge and the edges of the opening heaped up with pale unhealthy granulations. When a fistula is present, the external opening is large with purplish, overhanging edges and there is a copious, thin, creamy discharge. As in the non-tuberculous fistulae, there may be multiple external openings with extensive spread of the infection to the surrounding tissues of the ischiorectal fosse and perineum and eventually even to the buttocks. There is usually only one internal opening and this is generally superficial or between the sphincters. A fistula may complicate or can be complicated by a perirectal abscess; in the former instance, it may be of the blind external variety due to inadequate drainage of the original abscess.
Infection leading to fistula is generally supposed to originate from an infected anal gland. The infection usually occurs from the bowel lumen, the patient swallowing the sputum laden with tubercle bacilli. This is the commonest route of infection in patients suffering from pulmonary tuberculosis. The anal glands are at the base of the anal crypts and are located at the level of the dentate line. Most people have between six and eight such glands, which extend down into the internal sphincter and up to and including the intersphincteric groove. Obstruction of these glands leads to stasis, bacterial overgrowth, and ultimately abscesses that are located in the intersphincteric groove. These abscesses have several routes of egress, the most common of which are downward extension to the anoderm (perianal abscess) or across the external sphincter into the ischiorectal fossa (ischiorectal abscess) [16]. Less common routes of spread are superiorly up the intersphincteric groove to the supralevator space or in the submucosal plane. When the abscess is drained either surgically or spontaneously, persistence of the septic foci and epithelization of the draining tract may occur and lead to a fistula-in-ano.
The tubercle bacillus has a predilection for lymphoid tissue and so the infection usually spreads from the rectum through lymph channels and forms an abscess in the perianal tissues, though sometimes it may travel from the rectum to the perianal tissues by blood vessels or by direct extension [17]. The tubercle bacilli may also gain entrance into the blood stream from some extrarectal focus and may lodge in the fat of ischiorectal fossa to start there as an abscess. Fissures and abrasions round the anus may be infected by direct external inoculation or autoinoculation. These patients are commonly subjected to increased anal trauma, as both diarrhea and constipation are common in their disease. There is a diminution in the subcutaneous adipose tissue because of the patient’s poor general condition and thus the anal and perianal tissues can be traumatized easily. Lesions, such as abrasions or tears of the mucosa or skin at the mucocutaneous junction may be secondarily infected with local infective organism leading to an abscess.
This perianal abscess on rupture leaves a draining fistula. It rarely may be the result of a spread of the infection from the neighboring organs, which are the seat of tuberculosis, e.g., prostate, seminal vesicles, spine or sacroiliac joints [18].
In patients with a fistula, the abscesses are more likely to contain multiple different organisms. Fistula formation allows for increased bacterial invasion in the abscess. Most aerobic and anaerobic organisms isolated from such abscesses are of gastrointestinal tract and skin flora origin [19]. The incidence of gut-derived microorganisms including E. coli, B. fragilis, and Enterococci cultured from perirectal abscesses has been reported as between 70 and 80 %.
Clinical Presentations
Since these fistulae are commonly a complication of pulmonary tuberculosis, the majority of the patients are found to be in the third and fourth decades of life. Constitutional symptoms as anorexia, fever, weight loss, or malaise usually are not present. A previous history of perirectal abscess either drained spontaneously or surgically can usually be elicited. Patients often report a cyclical pattern of pain, swelling, and drainage. Moisture can cause skin irritation, excoriation, and pruritus (Fig. 20.2). Prior TB infection as a child, immunocompromised states such as human immunodeficiency virus/acquired immunodeficiency syndrome, immunosuppression with organ transplantation, travel to endemic areas, and immigration are important considerations while obtaining a medical history [20]. There is a general agreement that tuberculosis is rarely if ever primary in fistula; in more than 98 % of the cases the original focus is elsewhere. It may, however, be the initial sign of the disease, which should always be suspected with the appearance of a fistula in a susceptible individual from the situations mentioned above.
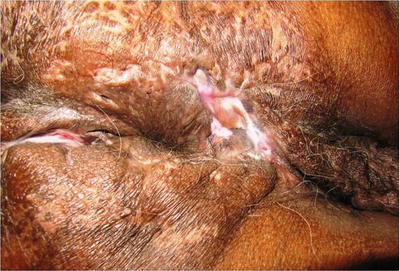

Fig. 20.2
Tubercular anal fistulae with scarring
A good digital examination is usually sufficient for the diagnosis and the treatment planning of anal fistula. There is no functional sign or preferred site that allows a tuberculous fistula to be distinguished from a cryptoglandular fistula. The external opening may be a pinhole or appear as an irregular ulcer of varying size (Fig. 20.3). The fistulous tract is patulous due to the absence of induration. The granulations are pale and flabby. A gaping external fistulous orifice with detached margins and highly vascular in nature is suggestive [21]. The discharge is frequently continuous and is thin and milky. These form the majority of the tuberculous fistulae and are labeled as the superficial or low variety. The deep or high tuberculous fistula forms a tract with little induration and often leads to a palpable submucous thickening high up (Fig. 20.4). Most authors report a higher frequency of complex tuberculous fistula forms (62–100 %) which sometimes puts the sphincter and continence at risk [22]. A long period of development and the recurrence of the fistula despite well-managed surgical treatment have been found in a majority of patients. The tract of the fistula and its relationship to the sphincter muscle can be investigated by probing and/or dyeing intraoperatively with the patient under anesthesia.
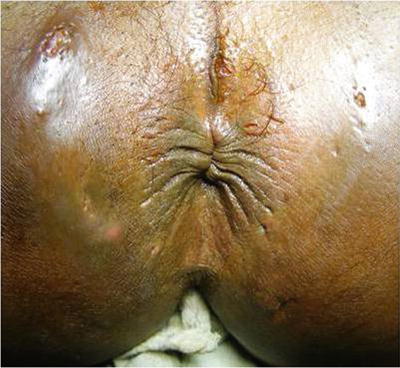
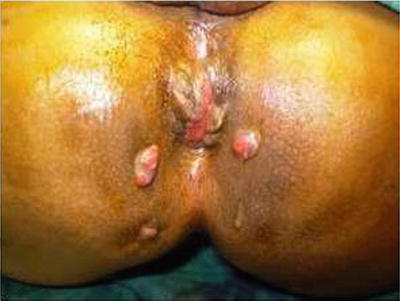

Fig. 20.3
Multiple pin-like openings of a tubercular fistula

Fig. 20.4
A horseshoe tubercular fistula
It is not necessary that all reported cases of anorectal tuberculosis are patients with old tuberculotic lesions or with active pulmonary tuberculosis in the lung. Diagnosis can be delayed, because physicians may neglect to perform a general physical examination in patients with a positive sputum smear and the typical chest X-ray. It is emphasize that all patients with pulmonary tuberculosis must undergo a complete physical examination. Chest physicians must remember that patients may not mention anal complaints if not asked. The possibility of tuberculosis must never be forgotten in the etiology of anal nodular and ulcerated lesions, regardless of the presence or absence of pulmonary tuberculosis [23]. The patient’s continence is another important facet that needs to be included, as well as any history of anorectal surgery.
Investigations
As tuberculosis of anal region is a rare entity and there is no characteristic clinical picture, it is very difficult to diagnose it preoperatively. The diagnosis of anal TB is difficult and may be missed for months or even years. Nevertheless, overlooking the diagnosis of perianal tuberculosis can result in chronic morbidity and often mortality, especially among immunocompromised patients. The physician must take a thorough history, look for acid-fast bacilli in the discharge from the lesion, carefully examine excised tissue, and culture for M. tuberculosis to make the correct diagnosis of this potentially curable disease [24]. Positive Siebert purified protein derivative of tuberculin test (PPD) supports TB infection, but a negative test does not rule it out.
The tuberculin skin test is one of the few investigations dating from the nineteenth century that is still widely used as an important test for diagnosing tuberculosis. It was developed by Koch in 1890, but it was Charles Mantoux, who described the intradermal technique currently in use in 1912. Short of demonstrating viable organisms in body tissues and fluids, the tuberculin skin test (TST) is the only method of detecting M. tuberculosis infection in an individual and is used in the diagnosis of TB in individual patients. The tuberculin most widely used is purified protein derivative (PPD), which is derived from cultures of M. tuberculosis. The reaction to intracutaneously injected tuberculin is the classic example of a delayed (cellular) hypersensitivity reaction [25].
A standard dose of five tuberculin units (TU) (0.1 mL) is injected intradermally and read 48–72 h later. A person who has been exposed to the bacteria is expected to mount an immune response in the skin containing the bacterial proteins. TST indurations >10 mm are considered positive, 5–10 mm intermediate; and <5 mm as negative [26].
The Mantoux test does not measure immunity to TB but the degree of hypersensitivity to tuberculin. There is no correlation between the size of induration and likelihood of current active TB disease but the reaction size is correlated with the future risk of developing TB disease. The test has a poor positive predictive value for current active disease. The formation of vesicles, bullae, or necrosis at the test site indicates high degree of tuberculin sensitivity and thus presence of infection with tubercle bacilli [27].
Negative tests can be interpreted to mean that the person has not been infected with the TB bacteria or that the person has been infected recently and not enough time has elapsed for the body to react to the skin test. There is disagreement about the role of Mantoux testing in people who have been vaccinated. The US recommendation is that TST is not contraindicated for BCG-vaccinated persons and that prior BCG vaccination should not influence the interpretation of the test [28].
Positive diagnosis of anal TB depends on histological or bacteriologic analysis. The typical histological lesion is the epithelioid and giant cell tubercle around a zone of caseous necrosis, but the pathognomonic presence of caseation is not constant and presents diagnostic problems, especially in the case of Crohn’s disease with anoperineal localization. And though this histological picture of granulomatous inflammation is characteristic of Mycobacterium infection, it can also be found in other disease entities such as sarcoidosis, leprosy, and systemic lupus erythematosus [29]. Tuberculosis, even when present, is frequently missed; in that too few sections are studied. Tissue for microscopical examination should be carefully selected and even then, there may be presence of such a large number of acute and chronic inflammatory cells, that the underlying tuberculosis may be missed. Diagnosis can also be done by looking for Koch’s bacillus in the anal lesions by direct examination (Ziehl–Nielsen stain) and culture. To overcome the slowness of the culture (3–4 weeks), new diagnostic techniques for TB have been proposed, in particular genomic amplification by polymerase chain reaction (PCR), which can detect the presence of the bacterial DNA in 48 h with high sensitivity and specificity when several samples are tested [30]. TB-nested PCR using pus or tissue specimens is a good alternative method with exquisite sensitivity and specificity for rapid and early detection of the disease. However, one of the disadvantages of PCR is its inability to detect whether the TB infection is biologically active or in its latent phase. New enzyme-linked immunosorbent assays for the diagnosis of digestive tract TB that appear to be highly specific and sensitive are currently undergoing evaluation [31]. Histological examination is mandatory if the patient has had or still has tuberculosis elsewhere in the body. Stool cultures are rarely useful and they are not routinely performed.
Transanal and endorectal sonography and other sonographic techniques may evaluate the nature and course of fistula [32]. This procedure is simple, fast, can be done in the same office visit, and provides information about the fistula track, the type, and complexity and whether the anal sphincter muscle is normal, scarred, or disrupted, as well as the presence of an abscess cavity. With the use of high-frequency linear or curved array probes on the perineum in both transverse and longitudinal planes, fistulous and sinus tracts, collections in the perineum, buttocks, scrotum, and labia can be assessed and followed in a retrograde direction to their connection with the anal canal. A high sensitivity of 96 % is reported for the detection of tracts, with a negative predictive value of close to 100 % on perianal sonography [33]. The advantage of endosonography is that it is easy and cheap to use, but it does depend to a high degree on the examiner’s experience.
Identified tracts appear on a sonographic scan as hypoechoic linear areas or fluid-containing tubular areas, depending on their size and activity, with contained particulate fluid or hyperechoic moving reflections created by air bubbles and pus. Highly complex trans-sphincteric tracts, which may extend to involve the deep tissues of the buttocks, the perineum, the scrotum in men, and the labia and vagina in women, can also be documented, along with horseshoe fistulas. Perianal fluid collections and abscesses present as oval hypo to anechoic masses, most often with a direct association with a fistulous tract [34]. This documentation of fluid collections formed by fistulas and of the relationship of inflammatory tracts to the sphincter mechanism is important for surgical treatment.
Sonography can identify the internal opening in the upper anal canal with a high accuracy in more than 90 % of patients. Further, 3D imaging may better map the course of a fistula than 2D imaging. However, for some authors, hydrogen peroxide or SH U 508A (Levovist, Schering) injection of the external opening and a 3D reconstruction during transrectal sonography may better demarcate small fistulous tracts, internal openings, and secondary extensions and may facilitate differentiation between an active fistulous tract and fibrotic tissue, thereby providing a dynamic depiction of perianal fistulas, although with some limits and possible pitfalls [35]. Anal ultrasound is operator dependent; scars and defects may confuse sonographic interpretation and might render delineation of the fistulous track difficult.
Cross-sectional imaging techniques can accurately identify deep abscesses and characterize complex fistulae. MRI is well suited for this examination, with almost no motion artifact, excellent contrast between muscles and fatty spaces, and multiplanar acquisition. The cryptoglandular anal fistulae are nonspecific in origin and are usually simple, whereas specific fistulae-like tuberculosis are at time complex. MRI appears useful in the cases with recurrent fistulae, when the secondary orifice is atypically placed, during a multistep treatment for complex fistulae, or when an anal stenosis forbids a clinical or ultrasound examination [36].
A good knowledge of the perineum anatomy is required for analyzing the fistula tracts. The muscle planes separate fatty spaces which have an important role in the spread of the disease: sub-mucosal space, marginal space, intersphincteric space, postanal space of Courtney, supralevator space, and the two ischioanal spaces on both sides of the anal canal, all have their role to play in the formation and spread of infection.
MRI examination is performed with an external phased array coil, where a canula is placed to identify the anal canal. The T2W sequences give the more interesting information, but the sequences with fat-suppression and gadolinium chelate injection are also very useful. Digital subtraction MR-fistulography is a new, noninvasive imaging technique for the detection of perianal fistulas and abscesses [37].
However, MRI is cost intensive, not always available, and its diagnostic value depends on technical conditions; however, it is to be preferred to endosonography for lesions distant from the anus. Other advantages of MRI are that it allows pain-free acquisition of images that can be evaluated independently of the examiner.
Because of the radiation burden, visualization of fistulas using contrast media like fistulogram and computed tomography (CT) is regarded as obsolete.
Although anal manometry may provide information about sphincter pressures, obtaining a fecal incontinence score will also yield clinically relevant information. Collectively, this information will help select the best choice for treatment and allows the surgeon to counsel the patient about expectations and probabilities of success.
The other investigative tools proposed for the diagnosis of tuberculosis includes the ELISA, Rapid Immunochromatographic assay (ICT Tuberculosis), Fluid adenosine deaminase estimation, Interferon gamma and the radiometric BACTEC system studies, The Xpert MTB/RIF assay, and QuantiFERON®-TB Gold test [38]. Fine needle aspiration cytology (FNAC) of the perianal lesions can also be performed as a supportive investigation.
The serodiagnosis of tuberculosis has long been the subject of controversy, as we still lack a test with widespread clinical utility. The poor sensitivity and specificity of commercial assays preclude their use as the sole means of diagnosis. All these assays use mycobacterial antigens adsorbed onto a surface. There is a high possibility of false positive and false negative reactions. The overall sensitivity of these tests for extrapulmonary tuberculosis is as low as 16 % [39].
The relatively low sensitivities and specificities of these serologic tests make them poor tools for the diagnosis of tuberculosis. For the EIA-IgA, the sensitivity reported was 74 % and the specificity was 68 % when a cutoff determined by a receiver operator characteristic curve was used. For the EIA-IgG, the sensitivity was 69 % and the specificity was 64 % [40].
Experience shows that a triad of fine needle aspiration cytology, AFB smear, and culture are cheaper, foolproof, and confirmatory when compared to costlier tests like TB IgG, IgM, or ICT tests.
Similarly, an elevated erythrocyte sedimentation rate (ESR) may be expected in patients with tuberculosis, but it has been found that one-third of patients with TB had a normal ESR at the time of diagnosis, and consequently there would seem to be little value in using ESR as a diagnostic test for tuberculosis.
Sometimes, a cluster of epidemiologic, clinical, histological, radiologic, and evolutive arguments can contribute to the diagnosis of tubercular anal fistula. These may include the origin and social class of the patient, previous history of TB, recurrent anal suppuration, weight loss, fever, night sweats, chronic dry cough, positive reaction to the tuberculin skin test, epithelioid granulomas without caseous necrosis on a sample from the lesion, associated evaluative pulmonary or digestive TB, and favorable and rapid response to antituberculosis treatment [41].
Differential Diagnosis
Differential diagnosis of anal TB must be done specially with Crohn’s disease, due to similar clinical, radiological, and endoscopic features [42]. While Crohn’s disease occurs in a relatively younger age group, TB is seen mostly in the elderly. Tubercular disease is predominantly a disease of the males; however, no gender is immune to Crohn’s. Tuberculosis presentations are nonspecific in general (recurring fistula, superficial ulceration); Crohn’s features are either typical (deep ulceration) or nonspecific (fissure, abscess, or recurring fistula). X-ray of the lungs would be usually normal in Crohn’s, while it would depict active lesions in the lungs or may show the sequel of the disease. Tuberculin test would obviously be positive in TB patients but would be negative in Crohn’s. The digestive tract in Crohn’s is frequently seen affected in the form of an ileocecal lesion, while it is rare to find such involvement in TB [43]. The histological features of Crohn’s disease is a granuloma without caseation or a nonspecific granuloma, while tubercular granulomas are more numerous, larger, often merging together and rich in multinucleated giant cells with or without caseation.
Culture of Koch’s bacillus is negative in Crohn’s while it may be positive or negative when observed in the excised tissue, urine, or gastric secretion. Relapses are common in Crohn’s disease even after a full course of therapy, while the tubercular infection is fully cured after the prescribed antitubercular therapy (ATT).
Other possible granulomatous diseases of the anus can mimic anal fistula, such as amoebiasis, actinomycosis (Fig. 20.5), reaction to a foreign body, sarcoidosis, syphilis (Fig. 20.6), and venereal lymphogranuloma with Chlamydia trachomatis. In rare cases, anoperineal TB can simulate cancer, particularly colloid cancer; here, histology is vital to make the correct diagnosis [44].
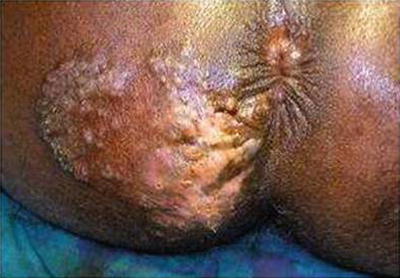
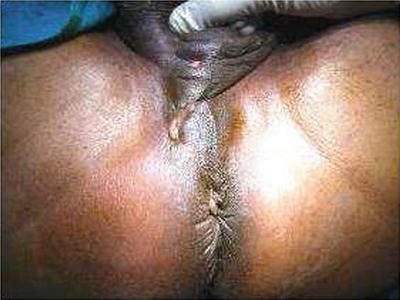

Fig. 20.5
Perianal actinomycosis with multiple pus discharging openings

Fig. 20.6
Anal syphilis with pus discharging opening in the perineum
HIV and Tuberculosis
Because of the absence of diagnostic symptoms and signs, the diagnosis of perianal tuberculosis can be much more complicated among HIV-positive patients, although it is considered an important marker of HIV infection. The development of active TB is dramatically accelerated by coinfection with HIV, which increases M. tuberculosis reactivation rates from 3–10 % per lifetime to 5–10 % per life-year [45].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








