Truncal Vagotomy with Antrectomy and Roux-En-Y Reconstruction
Kevin E. Behrns
Angel M. Caban
The introduction and evolution of acid-suppressive medications and the discovery of Helicobacter pylori as the cause of peptic ulcer disease have decreased markedly the number of patients who require elective surgical therapy. However, even though surgical therapy is indicated only in refractory cases of ulcer disease, it is essential that practicing general surgeons understand the pathophysiology and surgical options for the treatment of peptic ulcer disease. The early and important contributions of Dr. Lester Dragstedt identified the vagus nerve as an important stimulus to acid secretion. His experimental work provided the rationale for vagotomy as a mechanism to eliminate the direct cholinergic stimulus for acid secretion to the parietal cells. His work set the stage for the development of three variations of surgical vagal stimulus interruption: truncal vagotomy, selective vagotomy, and highly selective vagotomy. Since truncal vagotomy markedly alters gastric motility patterns, a gastric drainage or emptying procedure should be performed in conjunction with truncal vagotomy. Several surgical options for gastric drainage exist, but this chapter will focus on truncal vagotomy with antrectomy and Roux-en-Y reconstruction. It should be recognized that this operation was developed experimentally by Cesar Roux as a model of gastrojejunal anastomotic ulcer and, therefore, should be used only in refractory cases of ulcer disease. In fact, many surgeons preferably perform a gastroduodenostomy (Billroth I) or a gastrojejunostomy (Billroth II) for reconstruction after antrectomy because these operations represent more physiologic reconstructions of gastroenteric continuity.
Despite the limited application of this procedure, the indications for surgical intervention have not changed; surgery is often needed for intractable ulcers, bleeding, perforation, or obstruction. Importantly, the rate of emergency operations for complicated peptic ulcer disease has not changed since the introduction of acid-suppressive medications and antibiotics. Thus, emergent surgical therapy for complicated ulcer disease remains constant and requires that the general surgeon be up-to-date regarding new advances in surgical treatment. However, the emergent management of complicated peptic ulcer disease is beyond the scope of this work. The aims of this chapter are to describe the modern surgical approach to truncal vagotomy and antrectomy with Roux-en-Y reconstruction with emphasis on the indications, techniques, and postoperative outcomes. It is important to realize that the treatment of peptic ulcer disease is often individualized on the basis of the location of the ulcer, the inflammatory response, and the mode of presentation.
Truncal vagotomy and antrectomy with Roux-en-Y reconstruction is most often performed in an elective setting for recalcitrant peptic ulcer disease, and hence, a complete upper gastrointestinal tract evaluation should be completed. The evaluation should include an upper flexible endoscopy to identify the location of the ulceration, to rule out malignancy, and to test for H. pylori. In patients with prior upper gastrointestinal surgery a barium contrast study or computed tomography with oral contrast may be useful in delineating surgical anatomy. Serum gastrin level should also be obtained in patients with recurrent ulcers that are refractory to medical therapy and salicylate concentration should be determined for patients on nonsteroidal anti-inflammatory medications. Additional blood tests to assess the patient’s overall health and nutritional status may include serum electrolytes, a complete blood count, and nutritional markers such as albumin or pre-albumin.
A thorough history and physical examination is essential to assess the patient’s suitability for operative therapy with special emphasis on the history of previous surgery since most of these operations are now accomplished laparoscopically. A discussion with the patient regarding the benefits, risks, and long-term side effects of the operation is imperative. This conversation should include discussion of possible conversion to an open procedure, inadvertent or missed injuries, and postgastrectomy syndromes requiring lifestyle and dietary modifications.
In our practice the majority of the elective gastric resections are performed by a laparoscopic approach. Therefore, the laparoscopic approach will be presented in detail, but ultimately, the same operative steps are generally true for an open approach. In the unusual instance when the laparoscopic approach to this procedure is employed in patients presenting with bleeding or perforation, a low threshold for conversion to an open procedure should exist since visualization of important structures will be impaired in these cases.
Positioning
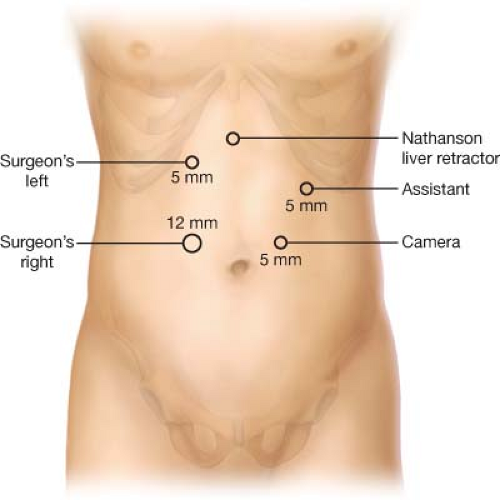
The patient is placed in the supine position and secured to the operative table on which a foot-board is applied to prevent patient slippage as a substantial portion of the procedure is performed in steep reverse Trendelenburg. The patient’s arms are usually abducted though they may be tucked at the patient’s side depending on the surgeon’s and the anesthesiologist’s preference. Typical trocar placement for upper gastrointestinal surgery is used as depicted in Figure 7.1. When an open approach is used, an upper midline incision from the xiphoid process to the umbilicus is often sufficient. Alternatively, a bilateral subcostal incision can be used for excellent exposure to the upper abdomen. However, if the patient has had previous abdominal surgery, adhesiolysis in the lower abdomen may prove difficult with the bilateral subcostal incision. Therefore, we prefer a midline incision.
Operative Technique
Following proper positioning, establishment of pneumoperitoneum, and port placement the operative approach is as follows:
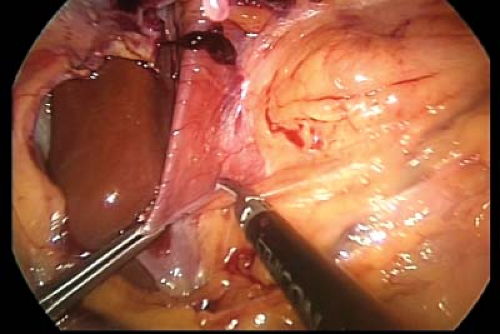
Dissection begins at the esophageal hiatus. The gastrohepatic ligament is incised until the right crus of the diaphragm is well visualized from apex to insertion (Fig. 7.2).
The phrenoesophageal ligament is divided from right to left anterior to the esophagus, until the left crus is visualized.
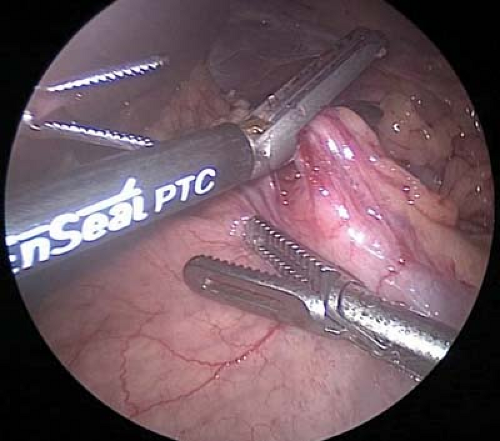
Circumferential dissection of the esophagus is performed to free approximately 4 cm of esophagus. The right and left vagus nerves are identified along the esophagus. The right vagus nerve is typically a relatively large cord-like structure and readily identified in the periesophageal tissue directly adjacent to the posterior surface of the esophagus. The more diminutive left vagus nerve is often directly applied on the anterior surface of the esophagus and less easily identified (Fig. 7.3).
Each nerve is then clipped and divided using a 5 mm clip applier and laparoscopic scissor. Approximately 1 cm of the nerve is removed and sent to pathology for histologic confirmation. To ensure complete vagotomy, 4 cm of the esophagus should be skeletonized of nerve-like tissue with particular attention to a criminal nerve of Grassi, which is the first branch of the right vagus nerve.
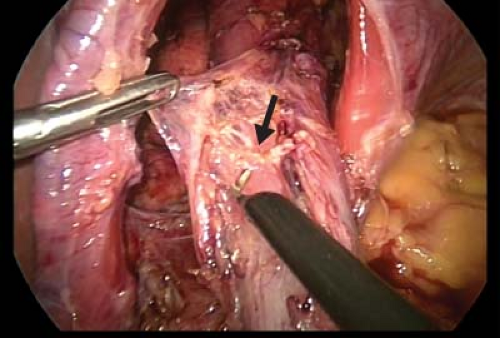
Figure 7.3 The anterior vagus nerve is mobilized from the esophagus (arrow). The nerve is then clipped and divided using a clip applier and laparoscopic scissors.
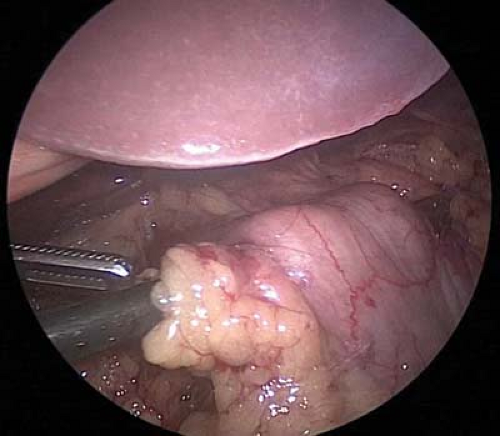
To perform the antrectomy the lesser sac is entered by dividing the gastrocolic omentum with a laparoscopic energy device. Once in the lesser sac, the omentum and the retrogastric attachments are divided toward the pylorus until the origin of the right gastroepiploic vessel is seen (Fig. 7.4). Circumferential dissection around this vascular pedicle is performed, and the pedicle is divided with a laparoscopic 60 mm linear stapler with a vascular load (2.5 mm staples) (Fig. 7.5).
Division of the gastrocolic omentum is continued orally until the selected point of gastric transection (Fig. 7.6). For antrectomy, approximately one-third of the stomach is removed.
Along the lesser curvature of the stomach, the right gastric artery is identified and divided using a clip applier and laparoscopic scissors or a stapling device as desired. Small arteries may also be divided with a laparoscopic energy device (Fig. 7.7).
A tunnel posterior to the first portion of the duodenum is created. In the presence of chronic inflammation this can be difficult and time consuming (Fig. 7.8). However, careful identification of surrounding structures will guide a safe dissection. Certainly, the location of the common hepatic artery and common bile duct should be known prior to division of the duodenum.
The duodenum can be divided just distal to the pylorus with a laparoscopic 60 mm linear stapler with 3.5 mm staples (blue load) (Fig. 7.9). The staple line should be examined carefully for defects; if integrity of the staple line is in question or significant inflammation or thickening is present, the staple line should be reinforced with suture.
The stomach is divided proximally to complete antrectomy, using a linear 60 mm stapler with 3.5 mm staples (Fig. 7.10).
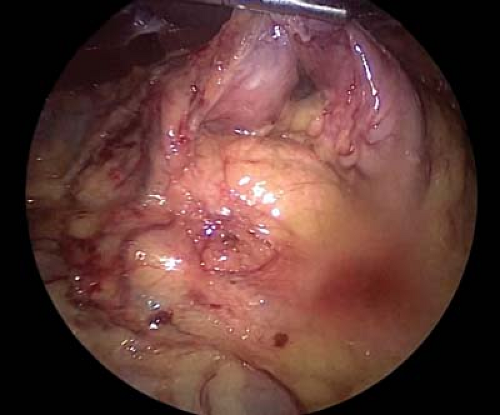
Figure 7.4 After dividing the gastrocolic omentum and entering the lesser sac, the origin of the right gastroepiploic vessels can be found near the pylorus.
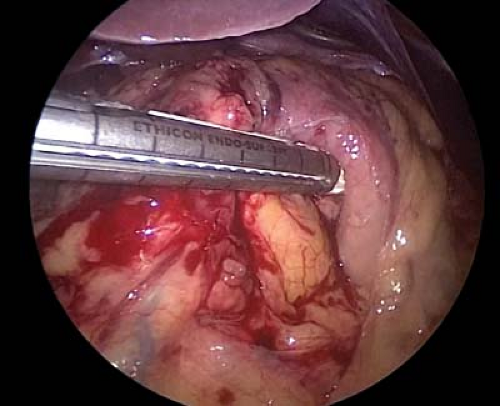
Figure 7.5 Division of the right gastroepiploic pedicle is performed with a laparoscopic linear stapler.
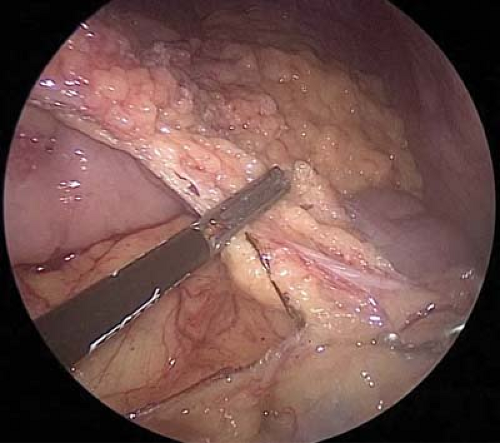
Figure 7.6 Cephalad division of the gastrocolic omentum using a laparoscopic energy device. Transections are continued proximally until the selected point of gastric transection.