SRMs are slow growing, and the average growth rate of RCC is 0.28 cm/year. They seldom progress to metastasize, which was reported in only 1 % of patients [3]. However, the growth rate of a renal mass does not predict malignancy with certainty as even tumours with zero growth rate have a malignant potential [4]. Neither clinical nor radiographic features can reliably categorize SRMs as benign or malignant and differentiate between indolent and potentially aggressive tumours.
So far, there is no established protocol for active surveillance of renal tumours. The tumour size may be an indicator of biological aggressiveness. In clinical T1b lesions (4–7 cm), the rate of tumour growth is 1.43 cm/year, and one out of nine patients develops metastasis. The risk of synchronous metastases increases to 22 % with each 1 cm rise in tumour size [5].
Renal mass biopsy with molecular analysis can assess the aggressive potential and guide in decision-making between active surveillance and active treatment.
16.2 Active Surveillance (AS)
Elderly patients and patients with co-morbidities with an incidentally detected SRM have a relatively low RCC-specific mortality. They can be considered for active surveillance. Active surveillance is defined as the initial monitoring of tumour size by serial abdominal imaging (ultrasound, CT, or MRI) with delayed intervention reserved for those tumours that show clinical progression during follow-up [6].
AS is a reasonable option in [2]:
1.
Patients with limited life expectancy
2.
Patients unfit for intervention
3.
Patients who do not desire immediate intervention
All the patients should be counselled about the small but real risk of cancer progression, the possibility to lose an opportunity for nephron-sparing surgery, the lack of curative therapies if metastases develop during the surveillance period and the limitations of renal mass biopsy in assisting the decision for active surveillance or active treatment.
16.3 Ablation Therapies
Many novel treatment modalities have evolved in the treatment of renal tumours: high intensity-focused ultrasound (HIFU), radiosurgery (CyberKnife), microwave thermal therapy (MWT), laser interstitial thermal therapy (LITT) and pulsed cavitational ultrasound (PCU). However, only two ablation therapies are recommended by expert panels for the treatment of renal tumours: cryoablation and radiofrequency ablation.
Ablative therapies are indicated in elderly patients (>70 years), small renal tumours (T1a), solitary kidneys, impaired renal function, post-surgical locally recurrent low-grade cancers and hereditary RCC (VHL, hereditary papillary RCC and tuberous sclerosis). Ideally, tumours treated with these techniques should be exophytic and distant from the hilum.
16.3.1 Radiofrequency Ablation (RFA)
It is a minimally invasive treatment option for localized renal masses in patients with high surgical risk. RFA is produced by an alternating electrical current of a frequency between 350 and 500 kHz through an electrode inserted in the tumour. The following sequence is observed [7]:
Ionic agitation → frictional tumour heating beyond 55 °C → coagulative necrosis.
It is important that the heat doesn’t exceed 100 °C, because that would produce an immediate local vaporization and carbonization limiting the electrical conductivity.
The resulting ablation zone generally encompasses the initial tumour with a 5–10 mm excess (Fig. 16.2a–d).
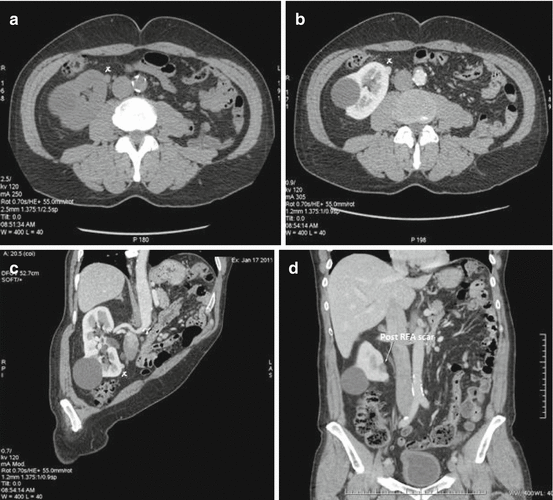

Fig. 16.2
(a–d) CT scan showing a small (1 cm) exophytic tumour (x) in a solitary right kidney in a 66 years man. This is a typical indication for ablative therapies. There is also a benign cyst. The tumour is very close to the upper ureter and the IVC (c). The patient had a laparoscopy-guided RFA. (d) Shows a post-treatment scar which is larger than the treated tumour
Relative contraindications: Large contact between the tumour and the renal excretory system (renal pelvis, upper ureter), the vessels or the bowels (anteriorly located tumours).
Absolute contraindications: Impaired coagulation, sepsis and severe mental retardation (non-compliance for regular follow-up).
16.3.2 Cryoablation (CA)
In the past, liquid nitrogen was used to perform CA. It has been replaced now by liquid argon, and the cooling can reach up to −185°. Cryotherapy causes tissue destruction using repeated freeze/thaw cycles. The use of multi-probes technique has the advantage of treating larger renal lesions than RFA. Unlike RFA, cryotherapy is also MRI compatible. MRI-guided ablation has the advantage of real-time guidance for probe placement, multiplanar imaging, exquisite soft tissue contrast and lack of ionizing radiation [8]. Also, there seems to be less damage to the urothelium, and the treated zone will be well defined by CT in the follow-up. Authors have also reported less pain associated with CA than RFA [9]. Cryoablation may be a treatment option for patients with high surgical risk and who are not candidates for observation. When cryoablation has failed, salvage surgery can be difficult due to fibrotic reaction within the perinephric space.
Both RFA and CA can be performed either by percutaneous approach with ultrasound or CT guidance or laparoscopically and are treatment options for patients with high surgical risk who want active treatment and accept the need for long-term radiographic surveillance after treatment. Renal biopsies are strongly recommended prior to ablation therapies to confirm and document the histopathology of the tumour. There is an increased risk of local recurrence, and a repeat renal tumour biopsy may sometimes be considered with the potential need for a second therapeutic intervention. Larger tumours, tumours with irregular shape or with an infiltrative appearance have an increased risk of recurrence when treated with thermal ablation.
16.4 Surgical Resection
RCC is resistant to radiotherapy and chemotherapy. Surgical extirpation remains the only curative therapy for RCC even in the era of targeted therapy. Various systemic treatment modalities such as immunotherapy were developed for advanced RCC, but targeted therapy has been accepted widely despite being costly. As for all malignant tumours, the treatment strategy is based on the stage of the disease. For operable tumours, there is a net demarcation between localized (stages I and II) and locally advanced tumours (stages III and IV).
1.
Stage I: (T1 N0 M0; ≤7 cm tumours):
The standard of care is partial nephrectomy [2, 10]. This can be performed by open-, laparoscopic- or robotic-assisted technique. Radical nephrectomy is recommended if partial nephrectomy is not technically feasible due to unfavourable tumour localization and characteristics (mid-polar endophytic tumour near the hilum). Other alternative modalities are thermal ablations: radiofrequency ablation and cryoablation. These are suitable for small cortical tumours, multiple, hereditary and bilateral tumours or in solitary kidneys.
Active surveillance is an alternative in patients who are at high risk for surgery and for those with a short life expectancy and tumour size smaller than 4 cm.
As stated by the American Expert Panel, there is no statistically significant difference in overall survival and cancer-specific survival observed between radical nephrectomy, partial nephrectomy and active surveillance for clinically T1a renal mass [2].
2.
Stage II: (T2 N0 M0; >7 cm tumours):
Primary treatment is radical nephrectomy, preferably by laparoscopy.
3.
Stage III: (T3 N0 M0; perinephric tissue invasion and venous thrombus):
Open radical nephrectomy is the standard of care. Laparoscopy can be considered. Tumour thrombectomy from renal vein or venal cava is indicated if involved.
4.
Stage IV: (T4, any T with N1 ± M1):
Locally invasive RCC may require aggressive surgical management with resection of adjacent organs. 40–60 % of patients may be cured by such aggressive surgical management [11].
When there is a solitary resectable metastasis, nephrectomy can be combined with metastasectomy. In patients with multiple metastases, a cytoreductive nephrectomy may be recommended prior to systemic therapy.
Systemic targeted therapy is recommended in patients unfit for surgery or unresectable tumour or mRCC [10].
16.4.1 Surgical Modalities and Approaches
16.4.1.1 Open Radical Nephrectomy (RN)
RN includes ligating the renal artery and vein, removing the kidney without opening the Gerota’s fascia, removing the ipsilateral adrenal gland and complete regional lymphadenectomy from ipsilateral crus of the diaphragm to aortic bifurcation [12] (Fig. 16.3).
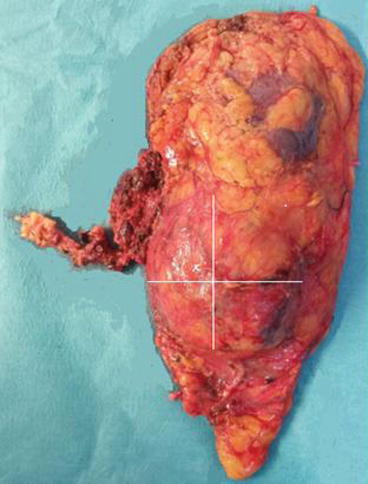

Fig. 16.3
Gross specimen of a radical laparoscopic left nephrectomy: there was a large tumour (x) involving all the lower half of the left kidney as well as few hilar LN
Thoracoabdominal, anterior subcostal and flank incisions are the most commonly used. Transperitoneal or extraperitoneal approaches are used depending on the size and location of tumour and patient habitus. Transperitoneal approach has the advantage of an excellent initial exposure of renal pedicle. Thoracoabdominal route is best for a large upper pole tumour. Extraperitoneal flank approach is preferable when the kidney is chronically infected, in obese patients, when there are multiple prior abdominal surgeries and if partial nephrectomy is considered. Details of the technique are beyond the scope of this book.
The oncological outcomes of open versus laparoscopic radical nephrectomy were found to be similar [13]. Laparoscopic radical nephrectomy (LRN) allows a shorter hospital stay and lower analgesic requirement. There is longer operative time in LRN, but less peri-operative blood loss and shorter convalescence time. There is no difference in complications rate and in post-operative quality of life (QoL) between the two techniques. No differences were also noted between retroperitoneal and trans-peritoneal approaches of LRN in oncological outcomes and QoL [14].
Adrenalectomy is not always necessary and is not routinely recommended. The tumour size is predictive of adrenal involvement but not its sole upper pole localization. Nephrectomies with and without adrenalectomy did not show any difference in overall survival at 5 and 10 years. Adrenalectomy is justified only if there is evidence of radiological invasion or intra-operative findings of involvement [15].
Lymph node dissection: Only 20 % of clinically detected lymph nodes by CT/MRI and intra-operative assessment are confirmed to be metastatic on histology. Furthermore, an EORTC study has shown that only 4 % of clinically N0 patients were found to have a positive lymph node in the final pathology [16]. These findings suggest that routine LND is clearly an over-treatment in the majority of N0 patients. Therefore, LND is not recommended in localized tumour without clinical evidence of lymph node invasion [10]. When there are clinically enlarged lymph nodes, LND can be performed for staging purposes or for local control.
16.4.1.2 Open Partial Nephrectomy (PN) or Nephron-Sparing Surgery (NSS)
This can be approached through a flank retroperitoneal approach or an anterior subcostal incision according to the surgeon’s preference. Whatever the approach, the kidney must be dissected free from the perirenal fat to leave only the fat overlying the site of tumour. Here again, details of the technique are beyond the scope of this book.
There is no difference in survival between laparoscopic PN (LPN) and open PN. The mean blood loss is generally lower, but operative time is longer in laparoscopic PN [17]. A study has noted a deeper decline in GFR in the immediate post-operative period in LPN but no difference in 3.6-year follow-up [18].
There are similar outcomes when comparing retroperitoneal versus transperitoneal LPN [19].
The cancer-specific survival of radical nephrectomy versus partial nephrectomy at 5 years is comparable for tumours less than 5 cm in size and a normal contralateral kidney. Although local recurrence rate is potentially higher in the partial nephrectomy group (Fig. 16.4a, b), a recent meta-analysis has shown that NSS had a significantly lower cancer-specific death rate compared with RN, but there was no significant difference in the rate of tumour recurrence and complications between the two techniques [20].
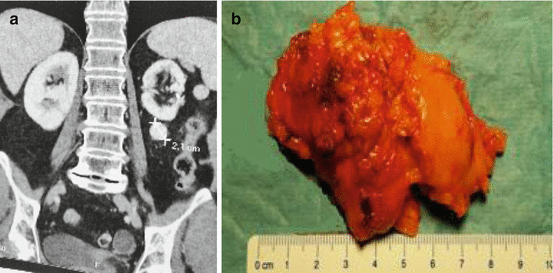

Fig. 16.4
(a, b) Local recurrence in the Gerota’s fascia 2 years after a laparoscopic left partial nephrectomy for a lower pole tumour. The recurrent lesion was estimated to be 2–3 cm diameter and was safely removed laparoscopically with enough peri-renal fat to ensure negative margins
In T1a RCC, radical nephrectomy, partial nephrectomy and radiofrequency ablation all showed a 5-year cancer-specific survival of 100 %.
The oncological outcome of partial nephrectomy supports that localized RCCs are best managed by nephron-sparing surgeries than by radical nephrectomy. However, in some patients with localized RCC, nephron-sparing surgery is not suitable because of locally advanced tumour growth, unfavourable location of tumour and a poor general condition. In these situations, radical nephrectomy is the rule [21].
16.4.1.3 Laparoscopic Radical Nephrectomy
Laparoscopic nephrectomy has become one of the commonest laparoscopic urologic procedures. Laparoscopy is the preferred approach in experienced centres for all indications of radical nephrectomy, and the larger tumour size is not considered a contraindication. The only limiting factor is the expertise of the urologist. Certain situations may be challenging such as large venous thrombus, adjacent organ infiltration and widespread metastases.
A transperitoneal approach has the advantage of a large working space with presence of familiar anatomical landmarks. Difficulties should be anticipated when there is history of multiple previous abdominal surgeries. On the other hand, a retroperitoneal approach performed over the triangle of Jean Louis Petit avoids the intra-abdominal viscera in the field and allows a rapid access to the renal artery, but the working space is limited, and the surrounding anatomy is less familiar.
Hand-assisted laparoscopic nephrectomy utilizes a 7–8 cm incision for the hand to retract, dissect and guide the laparoscopic tools. It is reported to have a shorter operative time.
16.4.1.4 Laparoscopic Partial Nephrectomy
This has emerged as an attractive minimally invasive alternative to open partial nephrectomy and is considered as the standard of care when facilities and expertise are available. It is a technically challenging procedure because of its intra-operative complexities and potential complications. It should be reserved for urologists with adequate prior laparoscopic experience.
Absolute indications of partial nephrectomy (open or laparoscopic) are tumour in a solitary kidney, patients with compromised renal function and bilateral renal tumours.
Relative indications are current or future threat to renal function such as diabetes, hypertension, contralateral kidney affected by renovascular disease, recurrent stone disease, cysts, ureteropelvic junction obstruction or other anatomical or functional abnormalities.
The elective indications include small renal tumours in the presence of a contralateral functioning kidney. Initially, elective partial nephrectomy was performed for exophytic tumours less than 4 cm size, but with the increasing experience nowadays, highly specialized centres regularly record partial nephrectomy for bigger size tumours, larger infiltrating tumours requiring heminephrectomy, centrally located and para-hilar tumours.
A pre-operative CT angiogram evaluates the tumour as well as the vascular anatomy. Intra-operative laparoscopic ultrasonography can also be useful to delineate the contour of an endophytic tumour during the resection.
Specific complications of nephron-sparing surgery include delayed haemorrhage and urinary leak, as well as the risk of renal function deterioration in longer warm ischemia time, especially if >30 min [22].
In Western Europe and North America, many centres routinely now perform robotic-assisted partial nephrectomy aiming at facilitating the endocorporeal dissection and stitching. Among other positive results, this technique helps to significantly reduce the warm ischaemia time [23].
Non-clamping techniques or selective arterial occlusion can be used for smaller exophytic lesions, with the purpose of avoiding warm ischemia insult to the remaining renal parenchyma.
16.4.1.5 Positive Surgical Margin After Nephron-Sparing Surgery
Positive surgical margins (PSM) were reported in 0–7 % of patients after open PN, in 0.7–4 % after laparoscopic PN and in 3.9–5.7 % after robot-assisted PN [24]. The thickness of healthy parenchyma surrounding the tumour is irrelevant as long as complete tumour removal is achieved. PSM is mostly associated with small and endophytic tumours. Gross evaluation of the resection margins by the surgeon is reliable, and routine frozen section is unnecessary [25]. A PSM raises the risk of local recurrence, especially in high-grade tumours. Large-scale data with nephrometry scores and long-term follow-up are lacking in this subject. Available data indicate that majority of patients with PSMs after partial nephrectomy will not experience disease recurrence. Moreover, prophylactic secondary nephrectomy of the renal remnant has shown residual tumour in only a minority (0–39 %) of patients with PSM [26]. Development of metastasis and cancer-specific survival was comparable to those in patients with negative surgical margins. Therefore, a surveillance strategy with adherence to vigilant follow-up is the best management option over surgical re-intervention [26, 27].
16.4.2 Management of Vena Caval Thrombus
Venous thrombus formation is a unique feature of RCC. It has been reported in 4–10 % of patients and was even found to involve the infrarenal vena cava [28, 29].
Although involvement of the IVC in renal cancer is generally not a vascular invasion by the neoplastic process but mostly an intraluminal extension of the tumour mass, such intravascular growth implies a heightened biologic behaviour of the tumour. Previous reports have shown that total resection of tumour, i.e. including any eventual venous thrombus, affords the best chance of cure and long-term survival when distant metastases are not present [29, 30].
Caval thrombus is one of the most technically challenging surgeries, but it is also rewarding for the urologist, because the 5-year survival rates, even in the face of intra-atrial tumour thrombus (Fig. 16.5a, b), are comparable to lesions confined to the kidney [29].
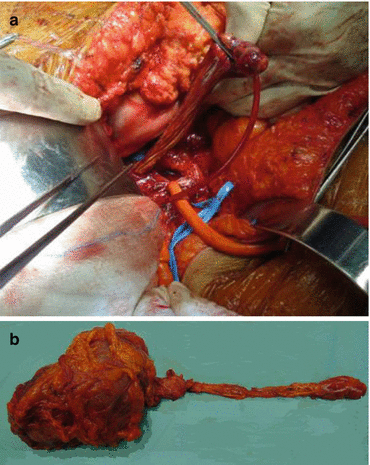

Fig. 16.5
(a, b) Cardiothoracic team removing a right atrial tumour thrombus from an RCC under extracorporeal circulation
A number of patients with caval thrombus are asymptomatic. But depending on the extent of occlusion, certain signs and symptoms may develop: lower extremity oedema, isolated right-sided varicocoele (irreducible), dilated superficial abdominal veins (caput medusae), proteinuria and even pulmonary embolism. Careful peri-operative planning is essential to optimize the outcome. Traditionally, venocavography was used as gold standard for the diagnosis of venacaval thrombus, but it is now rarely used as it does not show caval wall invasion and does not add any further information compared to US. MRI is considered the gold standard imaging for delineating the upper limit for tumour thrombosis as well as the extent of the caval wall invasion [30] (Fig. 16.6a, b). Alternatively, multidetector CT can be used with nearly equal performance [31].
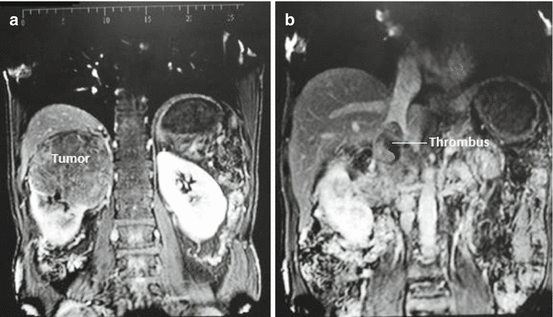

Fig. 16.6
(a, b) MRI showing a large right upper pole tumour (a) with venacaval thrombus (b) (Courtesy of Kurian George, Urology, The Royal Hospital, Muscat, Oman)
A multidisciplinary surgical team approach involving vascular, hepatobiliary and cardiothoracic surgical teams is needed in addition to the urologist.
In the presence of a large renal tumour with venacaval thrombus, the following surgical principles apply [30, 32]:
1.
Complete resection of the tumour and caval thrombus
2.
Mobilization of inferior vena cava (IVC) and the contralateral renal vein (RV)
3.
Vascular control of the contralateral RV and IVC proximal and distal to the thrombus
4.
Restoration of hemodynamic stability by vascular bypass
5.
Reconstruction of IVC if more than 50 % of the wall is involved by tumour
Various classification systems were developed to characterize the venacaval thrombi on the basis of their cranial extent (Neves, Novick, Hinman and Mayo classification systems). The most commonly used system and the appropriate corresponding management are given in Table 16.1.
Table 16.1
Classification and management of IVC tumour thrombus in RCC
Levels | TNM stage | Cranial extend of tumour | Surgical planning | Incision | Thrombectomy technique |
|---|---|---|---|---|---|
Level I | T3a | Renal vein extending <2 cm within IVC | Experienced urologist alone | Flank | Nephrectomy + RV thrombectomy, Satinsky parallel to wall of IVC to include only the ipsilateral RV ostium |
Level II | T3b | Infrahepatic | Vascular | Flank vs. Chevrona < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




