Fig. 1
The ReShape Medical ReShape Duo Balloon inflated in the stomach
Table 1
Characteristics of intragastric balloons evaluated in humans
Name | Company | Design | Fill | Placement/retrieval |
|---|---|---|---|---|
ORBERA (formerly Bioenterics Intragastric Balloon) | Apollo endosurgery, Austin, TX | 500 ml silicon balloon | 500 ml saline with methylene blue | Endoscopic/endoscopic 6-month duration |
ReShape Duo balloon | ReShape Medical, San Clemente, CA | Two 450 ml silicon balloons tethered to a flexible silicone shaft | 375–450 ml saline with methylene blue | Endoscopic/endoscopic 6-month duration |
Heliosphere bag | Helioscopie medical implants, Vienne, France | 550 cm3 polyurethane and silicone sphere | 550 ml air | Endoscopic/endoscopic 6-month duration |
Spatz adjustable agstric balloon | Spatz FGIA, Jericho, NY | 800 ml silicon balloon mounted on a catheter, adjustable after initial placement | 500–800 ml saline | Endoscopic/endoscopic 12-month duration |
MedSil balloon | MedSil, Moscow, Russia | 700 ml silicon balloon | 400–700 ml saline | Endoscopic/endoscopic 6 months |
Silimed gastric balloon | Silimed Industria de Implantes, Rio De Janeiro, Brazil | 650 ml silicone balloon | 632 ml saline, 20 ml Iopamiron contrast, 10 ml 2 % methylene blue | Endoscopic/endoscopic 6 months |
Obalon | Obalon therapeutics, Carlsbad, CA | 250 ml balloon, up to 3 placed sequentially | Nitrogen gas | Swallowed pill/endoscopic 3 months |
IGBs have been shown to be effective at inducing weight loss greater than lifestyle therapy alone or pharmacotherapy alone. A meta-analysis with 3608 subjects demonstrated an estimated mean total body weight loss of 12.2 % (14.7 kg) with at least 12 weeks of therapy with the ORBERA balloon [73]. A randomized controlled cross-over trial demonstrated superiority of IGB’s to pharmacotherapy. At balloon retrieval (6 months) total weight loss with IGB was 14.5 ± 1.2 % compared to 9.1 ± 1.5 % in the sibutramine group, p< 0.05 [74]. Moreover, at 1 year 50 % of patients who received an IGB plus lifestyle therapy maintained ≥ 10 % total body weight loss compared to 35 % of patients in the sibutraine group.
Long-term weight loss data after one balloon placement is sparse. One studyevaluated patients 60 months after balloon removal; however, only 195/474 patients returned for 60-month evaluation [75]. Total weight loss was 27.3 ± 9.6 kg and 7.26 ± 5.4 kg at the time of the balloon removal and at 60 months, respectively, with 23 % maintaining percent excess weight loss > 20 %. Although, the maintenance of some weight loss long-term is encouraging, investigators have evaluated the repeated use of IGB for long-term weight loss therapy. Genco et al. reported 100 obese patients who were randomized to receive an IGB for 6 months followed by lifestyle therapy alone or IGB followed by another IGB placement 1 month later for 6 months [76]. Thirteen-month percentage excess weight loss was 51.9 ± 24.6 % in the two consecutive IGB group and 25.1 ± 26.2 % in the IGB then lifestyle therapy alone group. A subsequent study by Genco et al. followed 83 patients for 6 years, and replaced the IGB after the patients regained ≥ 50 % of the weight lost from the previous IGB placement [77]. All patients required a second balloon placement after an average of 12 months (range 1–55 months). Eighteen patients required a third balloon and one patient required a fourth balloon. Initial BMI prior to the first balloon placement was 43.7 kg/m2, and 37.6 kg/m2 at 76-month follow-up. However, 18 patients underwent bariatric surgery instead of continuing balloon placement between 12 and 72 months after the first balloon was removed.
The rate of serious complications in a large case series of 2515 patients was < 3 % and including gastric perforation, gastric or small bowel obstruction, esophagitis, and gastric ulceration [78]. Two deaths related to gastric perforations occurred. Common mild to moderate adverse reactions in the first few days after balloon placement include nausea, vomiting, and abdominal pain which occur in most patients for the first few days after device placement. This was treated with oral medication and hydration in an outpatient setting in most patients, and typically resolved in a few days.
Taken together, these data suggest that the current generation of IGB has lower complication rates and better efficacy than the first generation of IGB. Although acute postplacement nausea, vomiting, and abdominal discomfort occurs, it is controlled with oral medications and dietary changes in most patients. IGB placement is an effective tool for short-term weight loss, and is superior to both lifestyle therapy alone and pharmacotherapy. Long-term weight loss does occur in some patients after IGB removal, but repeated balloon placement based on weight regain may be a better strategy for long-term weight control. Additional research is needed to address these long-term management questions.
Duodenal Jejunal Bypass Liner
The duodenal jejunal bypass liner (DJBL; EndoBarrier, GI Dynamics, Boston, MA; Fig. 2) is an impermeable flouropolymer duodenal jejunal liner that extends 60 cm into the small bowel. The device is placed endoscopically and anchors in the duodenal bulb via a nitinol anchor with barbs that allow for reversible fixation. Ingested nutrients flow into the liner, which prevents contact with the intestinal mucosa or biliary secretions until the liner ends in the mid-jejunum, creating a functional bypass of the duodenum and proximal jejunum which mimics the biliopancreatic limb of a RYGB .


Fig. 2
The GI Dynamics EndoBarrier, a duodenal jejunal bypass liner
Multiple studies have demonstrated the efficacy of bariatric surgery in treating type 2 diabetes, including two recent randomized control trials of bariatric surgery compared with the intensive medical and lifestyle therapy for the treatment of type 2 diabetes [56, 79]. The RYGB was superior to medical/lifestyle intervention [56, 79] and laparoscopic adjustable gastric banding [56] at inducing partial or complete remission of type 2 diabetes. Animal models suggest that jejunal nutrient sensing after duodenal exclusion plays a role in the early improvement in glycemic control after duodenal jejunal bypass surgery [80], and support the hypothesis that exclusion of the biliopancreatic limb has weight loss independent effects on multiorgan insulin sensitivity and glucose metabolism. However, the human studies are less clear and confounded by the effect of calorie restriction and altered metabolic responses to a meal which occurs immediately after surgery [81–84] .
Initial experience with the DJBL demonstrated the device to be superior to lifestyle therapy in multiple short (12–24 weeks) single arm and randomized controlled trials for weight loss [85–87]. Subjects in the DJBL group achieved 19.0–23.6 % excess weight loss (EWL) compared to 5.3–6.9 % EWL in the control groups. A longer therapy has subsequently been shown to produce more weight loss, and a single arm trial with 52 weeks of therapy in 39 subjects demonstrated 22.1 ± 2.1 kg weight loss (19.9 ± 1.8 % total body weight loss) [88]. A sham controlled trial for preoperative weight loss demonstrated lower weight loss 11.9 ± 1.4 % compared with 2.7 ± 2 % excess weight loss in the DJBL (n = 13) and control groups (n = 24), respectively (p < 0.05) [89]; however, another 24 week randomized sham-controlled trial demonstrated superiority of the DJBL over sham control for decreasing HbA1c in patients with type 2 diabetes (− 2.4 ± 0.7 % and − 0.8 ± 0.4 % in the DJBL and control groups, respectively, p < 0.05) [90]. Similar decreases in HbA1c were also seen in a single arm 24-weeks trial (HgbA1c 8.4 ± 0.2 % to 7.0 ± 0.2 % from baseline to end study, p < 0.01)[91], but more importantly, glycemic control after a liquid mixed meal test improved 1 week after implantation before any significant weight loss occurred, suggesting that the effect on glucose control may be weight loss independent. Subsequently, longer studies have been performed out to 52 weeks including a study in obese diabetic subjects with a 52-week HbA1c reduction of − 2.3 ± 0.3 % in 13 subjects [92]; and in subjects with lower BMI (30.0 ± 3.6 kg/m2) with a week 52 HbA1c reduction from 8.7 ± 0.9 % to 7.5 ± 1.6 % (baseline to end study, respectively, p = 0.004) with only 6.5 ± 4.1 kg weight loss [93] .
A few serious adverse events have been reported and no deaths have occurred in relation to the DJBL. One duodenal perforation requiring laparoscopic closure has been reported [94]. Of note, 17–40 % of subjects enrolled in the studies mentioned above had early device removal predominantly due to complications including: gastrointestinal bleeding, abdominal pain, nausea and vomiting, anchor migration, or obstruction. However, in most of these cases, the issues resolved with the removal of the device.
The efficacy data and relative safety of this device are promising as an effective treatment for obese subjects and potentially even overweight subjects with diabetes. A large multicenter randomized sham controlled trial of this device in diabetic subjects is currently underway in the USA. This will provide crucial information on efficacy and safety end points as well as further information on any persistent effects of the DJBL on glucose control after the device has been removed .
Intragastric Suturing to Alter Gastric Anatomy
A number of different approaches have been studied for per-oral gastric volume reduction. Transoral gastric volume reduction in the TRIM (transoral gastric volume reduction as intervention for weight management) trial utilizing the RESTORe suturing system (Bard/Davol, Warwick, RI) to plicate the anterior and posterior walls of the stomach was first reported in humans in 2010 [95]. This procedure reduces gastric volume by using a plication device to approximate the anterior and posterior gastric walls, and is thought to be a restrictive procedure. A total of 18 patients received an average of six plications; however, only 14 subjects completed 12 months of follow-up demonstrating a weight loss of 11.0 ± 10.0 kg (excess weight loss 27.7 ± 21.9 %). No serious adverse events occurred, but plication was only successful in 16 patients and at the 12-month endoscopy, all sutures had spontaneously released in five subjects [95].
Transoral gastric volume reduction has also been reported with the overstitch endoscopic suturing system (Apollo Endosurgery, Austin, Tx; Fig. 3) in a single center pilot study. The full thickness endoscopic suturing device was used to create an endoscopic sleeve gastroplasty in four subjects [96]. The weight loss data are not available; however, a repeat endoscopy at 3 months in two patients revealed intact endoscopic gastric sleeves with only a small portion of the sleeve open in the gastric fundus of one subject. No serious adverse events occurred, but nausea and abdominal pain occurred in three patients, with one patient admitted for conservative therapy. All symptoms resolved by 72 h post-procedure. Since the overstitch endoscopic suturing system has been approved for intragastric suturing by the Food and Drug Administration (FDA), select centers in the USA and abroad have now started offering this therapy.
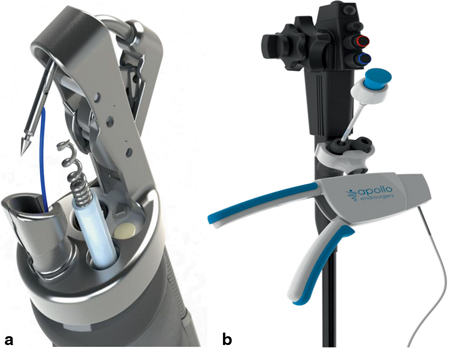

Fig. 3
The Apollo Endosurgery Overstitch tip with suturing arm and tissue helix on the end of an endoscope (panel A) and Apollo Overstitch handle attached to dual lumen endoscope (panel B)
The IOP (USGI Medical, San Clemente, California, USA, Fig. 4) has also been used to create gastric tissue plications as a primary weight-loss procedure (primary obesity surgery endoluminal, POSE) . This procedure involves creating tissue plications in the fundus and in the distal gastric body, which is thought to impair both gastric accommodation with a meal and delay gastric empyting. This increases satiation with a meal and satiety between meals, reducing food intake to produce weight loss. Results of a single arm, single center study of 45 patients was published in 2013. The 6 month data were only available for 27 of the subjects and demonstrated 16.3 ± 7.1kg (15.5 ± 6.1 %) total weight loss [79]. No serious adverse events occurred. Minor post-operative adverse effects included sore throat, abdominal pain, nausea, and chest pain; however, these typically resolved within 24 h and did not require additional hospital stay. A multicenter randomized sham-control trial is currently underway in the USA to further investigate the effectiveness of this procedure.
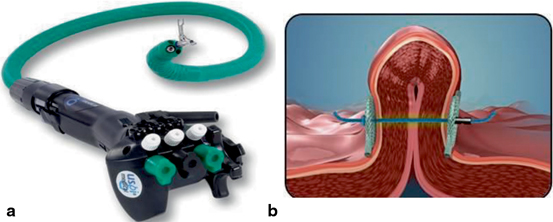

Fig. 4
The USGI Medical IOP Transport (panel A) and tissue plication with suture anchors (panel B)
Transoral mucosal excision with sutured gastroplasty, which employs the use of both a tissue excision device and suturing device to create a gastric pouch similar to the pouch created with laparoscopic adjustable banding, has been demonstrated to be feasible in four patients [97]. The BMI ranged from 39–61 kg/m2, and percentage excess weight loss ranged from 0–68 % at 24 months. One subject had evidence of perforation on chest x-ray on post-operative day 1; however, no perforation was detected on a combined laparoscopic/endoscopic procedure performed that day. The patient did recover, but an endoscopy performed at 6 months demonstrated a loose gastroplasty with no food restriction.
Although gastric volume reduction is still in its infancy, the initial data is promising as a means to alter gastric anatomy without the need for external incisions. Further studies are necessary to evaluate the efficacy and long-term durability of these procedures.
Aspiration Therapy
Aspiration therapy involves the use AspireAsisst™ system (Aspire Bariatrics, King of Prussia, PA, Fig. 5) to remove up to 30 % of the calories consumed in a meal for weight loss. This is done through a gastrostomy tube called an A-Tube, which is placed with the pull technique commonly used for placement of percutaneous endoscopic gastrostomy tubes. This is capped with a skin port that allows for connection to the companion, which is a hand held device that allows for bidirectional flow of fluid either into the stomach or out of the stomach controlled by an external lever. Patients infuse tap water to aid with aspiration of gastric contents, and then switch the direction of the flow to allow gastric contents to drain out into the toilet. Data from one randomized control pilot study demonstrated superior weight loss in the aspiration therapy group (n = 10) compared to the lifestyle therapy only group (n = 4) at 1 year (18.6 % ± 2.3 % and 5.9 % ± 5.0 % total body weight loss, respectively; p = 0.021). Lifestyle therapy consisted of 15 individual therapy sessions plus quarterly group sessions. 7 out of 10 subjects in the aspiration therapy group completed 2 years of therapy and were successful in maintaining their weight loss; 21.2 % ± 2.8 % total body weight loss at 1 year and 20.1 % ± 3.5 % total body weight loss at year 2, respectively; p = 0.547[98]. Multiple psychological evaluations were performed in these subjects, which demonstrated no adverse effects of this therapy on eating behaviors. Indirect measurements of food consumption also suggest that subjects did not eat more to compensate for calories that were aspirate. No serious adverse events occurred; however, abdominal pain in the first 4 weeks after device placement and peristomal skin irritation were common. Pain after the first 4 weeks of device placement was common with the initial A-Tube design used in the study. The A-Tube was modified during the trial to an all-silicone tube. The sustained weight loss and safety profile of the aspiration therapy support further evaluation of this therapy. More recently, preliminary results of a pilot trial conducted in the Czech Republic on six super obese patients (BMI 59.5–71.9 kg/m2) were presented as an abstract [99]. Weight loss at 3 months was 15.5 kg. Only two subjects had reached the 12-month time point at the time of the abstract, with an average weight loss of 42 kg. Only three minor adverse events occurred, procedure success rate was 100 %, and all subjects were still using the therapy at the time of the abstract presentation. These early results suggest that the aspiration therapy may be a good long-term treatment for obesity even in the super obese population. Further studies are currently underway in Europe for obese and super obese subjects, and in the USA, a multicenter trial is also being conducted in obese subjects.
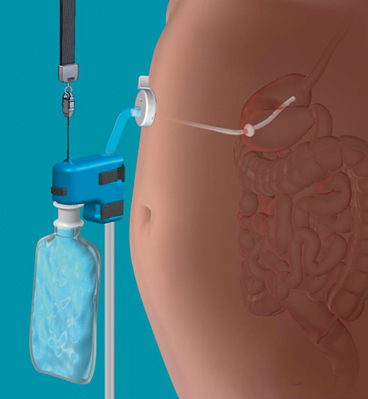

Fig. 5
The components of the Aspire Bariatrics Aspire Assist™ System assembled for aspiration
TransPyloric Shuttle
The transpyloric shuttle (TPS, BAROnova Inc., Goleta, CA; Fig. 6) is a spherical bulb that is connected to a smaller spherical bulb by a flexible tether. The larger spherical bulb intermittently obstructs the pylorus, which is thought to delay gastric emptying and promote satiation resulting in early termination of a meal. One pilot trial has been reported in 20 subjects (BMI 36.0 ± 5.4 kg/m2) [100]. The subjects were divided into two groups; a 3-month cohort (n = 10) with the TPS removed at after 3 months and a 6-month cohort (n = 10) with the TPS removed after 6 months. Subjects in the 3-months cohort achieved 8.9 ± 5.0 % total body weight loss while the 6 month cohort achieved 14.5 ± 5.8 % total body weight loss. No serious adverse events occurred and the TPS was generally well-tolerated without nausea or abdominal pain even immediately after device placement. However, ten subjects developed gastric ulcerations, and two of those subjects required early device removal (one in the 3-month cohort and one in the 6-month cohort).
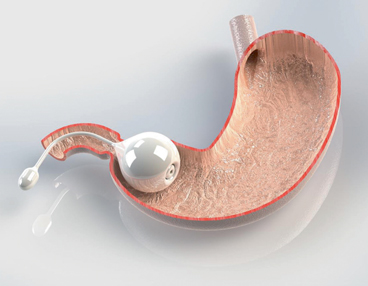

Fig. 6
The BAROnova TransPlyoric Shuttle in the pyloric position
Smart Self-Assembling Magnets for Endoscopy
Creation of gastroenteric anastomoses with smart self-assembling magnets for endoscopy has been shown to be technically feasible in an animal model [101]. The procedure described required advancing the gastroscope into the peritoneal space to secure the small bowel for placement of one set of the magnets. It is possible that this technique may be modified to be used in a human model. This presents a potential new mechanism for permanently bypassing the duodenum and proximal jejunum for weight loss and treatment of diabetes without the need for external incisions .
Summary
Primary EBT fills an important gap in the treatment of obesity . Multiple devices currently being studied are poised to meet FDA requirements for approval. While these new technologies provide an important step forward for obesity treatment, they do present new challenges for gastroenterologists. First, these devices and procedures were studied in conjunction with the lifestyle therapy. Endoscopists who wish to use these therapies for their patients will be required to develop an aftercare program that includes weight management program or partner with a reputable bariatric program. Secondly, the EBTs presented in this chapter have different mechanism of actions, and each one likely benefits a subpopulation of obese patients. The endoscopists will need to develop a skill set that allows them to determine which therapy best suits each individual patient based on a variety of patient characteristics. Third, these devices and procedures will likely not be covered by third party payers when they are initially introduced into the market. This will force the endoscopists to explore new financial models currently in place for other self-pay medical services .
Conclusions
Bariatric endoscopy for both weight regain after RYGB and for primary therapy is rapidly progressing into an important part of obesity therapy. A few therapies are already available for endoscopists, and multiple therapies will likely be commercially available in the next few years. While this opens the door to the endoscopists providing important therapies for obesity , it also presents new challenges that endoscopists must face in order to use these therapies successfully.
References
1.
2.
Katzmarzyk PT, Reeder BA, Elliott S, Joffres MR, Pahwa P, Raine KD, et al. Body mass index and risk of cardiovascular disease, cancer and all-cause mortality. Can J Public Health. 2012;103(2):147–51. PubMed PMID: 22530540.
3.
4.
5.
6.
7.
Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006 Nov;244(5):734–40. PubMed PMID: 17060766. Pubmed Central PMCID: 1856611.CrossRefPubMedCentralPubMed
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








