Necrotizing enterocolitis (NEC) is a devastating condition characterized by diffuse intestinal inflammation and necrosis in preterm infants. It is the most common gastrointestinal emergency in the neonatal intensive care unit and is associated with significant morbidity and mortality. Primary risk factors include prematurity and low birth weight. Although the pathogenesis of NEC is complex and not entirely understood, it is known that an interplay between immature intestinal immune responses and the process of bacterial colonization is required for the development of this disease.
Key Points
- •
Necrotizing enterocolitis (NEC) is a devastating disease characterized by diffuse intestinal inflammation and necrosis, with few effective preventive and therapeutic options.
- •
The primary risk factors for the development of NEC are prematurity, immature intestinal immune responses, and bacterial colonization.
- •
Several functional characteristics of the immature intestine render the preterm infant ill-equipped to manage extrauterine bacterial stimulation.
- •
Through complex mechanisms, probiotic bacteria modify innate immune responses of the immature intestine, suggesting an effective preventive strategy against NEC.
- •
Clinical trials demonstrate effectiveness of probiotics in reducing the incidence of NEC as well as data to support the safety of this practice.
The pathogenesis of disease is complex and therefore an active area of research. A critical interplay between bacterial colonization during enteral nutrition initiation and hypoxia-related injury, occurring in a susceptible host, results in an inappropriate, exaggerated inflammatory response and subsequent intestinal necrosis. Maturity of intestinal innate immune response is directly related to gestational age, placing the preterm infant at an increased risk for development of disease. However, not all premature infants develop NEC, suggesting specific susceptibility beyond gestational age and birth weight. Several additional risk factors have been described, including mode of delivery, source of nutrition, NICU environment, and early exposure to antibiotics. As the process of bacterial colonization is required for the development of NEC, delaying enteral nutrition initiation has been attempted using prolonged parenteral nutrition protocols. This approach may promote intestinal atrophy and compromise feeding success later and has increased risks of infection and prolonged hospitalizations.
If the development of NEC was strictly related to intestinal immune immaturity, one would expect disease development to be solely related to gestational age; however, disease presentation is typically between 7 and 14 days after delivery, regardless of gestational age at birth. This timeline parallels the one required for colonization with anaerobes. Close clinical monitoring is critical during feed initiation, because the signs of developing intestinal inflammation and necrosis may be nonspecific and may progress rapidly. The classic triad at presentation includes (1) feeding intolerance, such as increased gastric residuals or bilious gastric drainage, (2) abdominal distention, and (3) bloody stools. Intestinal perforation, pneumatosis intestinalis, portal venous gas, peritonitis, and hemodynamic instability are signs of severe, rapidly progressing disease and are poor prognostic factors. In addition to the using of abdominal plain radiographs, abdominal sonography may be useful in detecting earlier signs of developing disease, such as increased bowel wall echogenicity and thickness as well as early perfusion compromise.
Whether a patient requires medical or surgical management depends on several factors including the severity of disease at presentation and underlying comorbidities, such as baseline ventilatory requirements and cardiovascular status. Most patients respond to medical management (ie, bowel decompression, intravenous hydration, broad-spectrum antibiotics, electrolyte repletion), whereas approximately 30% require urgent surgical resection of necrotic bowel. Infants who survive are at risk for stricture development and those requiring surgical intervention face long-term complications related to short-bowel syndrome, including malabsorption, hyperalimentation dependence, and cholestatic liver disease. In addition to intestinal morbidity, neonates who develop NEC have an increased risk of long-term neurodevelopmental complications, particularly those with severe disease, extremely low birth weight (<1000 g), and associated late bacteremia.
In addition to nearly $1 billion in annual expenses required for the acute care of NEC patients in the United States, $1.5 million for every 5 years of ongoing outpatient care of survivors is necessary for management of chronic morbidities. Given the aggressive nature of this disease and suboptimal therapeutic options, the clinical focus has appropriately turned to preventive strategies. Currently, breast milk is emphasized as an effective preventive approach in this vulnerable population. In addition to increased concentrations of protective oligosaccharides in breast milk, particularly in the first 15 to 20 weeks of life, stools of infants who are fed breast milk have a significantly higher concentration of lactic acid and lower pH ( P <.05), compared with formula-fed infants. Although a meta-analysis by McGuire and colleagues demonstrated the protective effects of breast milk against the development of NEC, providing breast milk to all preterm infants is extremely difficult to accomplish in the United States, without an established breast milk banking system. Proposing additional preventive strategies, such as probiotic bacteria, requires an understanding of the multiple complexities, which place preterm infants at risk for developing NEC.
Host susceptibility in preterm infants
Immaturity of the innate intestinal immune system renders the premature infant who is poorly equipped to manage the immunologic challenges of extrauterine life. The immune system before delivery is T H 2-predominant and allows avoidance of maternal rejection of the fetus. The process of postnatal tolerance is dependent on a transition from T H 2-predominant to T H 1-predominant immunity and is influenced by the intestinal microbiome. The initial stages of bacterial colonization, occurring during the process of delivery and initiation of enteral nutrition, modify intestinal immunity and allow tolerance of further microbial stimulation. Specifically, the interaction between nutrients and early colonizing bacteria influences T-lymphocyte development and maturation. Recognition of luminal bacterial components by T-lymphocytes is critical to the balance between tolerance and development of gastrointestinal illness.
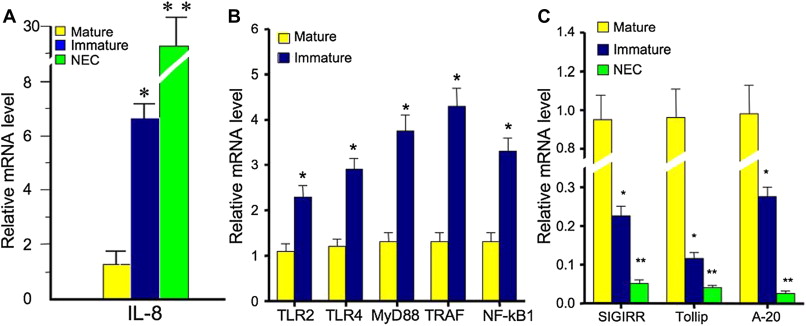
Maturation of intestinal innate immune responses occurs in a predictable manner in mature neonates; however, this process is poorly regulated in the setting of prematurity. As a result, the immature intestine shows an exaggerated inflammatory response after exposure to bacteria and bacterial products, compared with the mature intestine. Interleukin (IL) 8 is a chemokine, which has the ability to recruit neutrophils to areas of inflammation and therefore plays a critical role in the inflammatory response. Nanthakumar, and colleagues illustrated a significantly higher expression of IL-8 messenger RNA (mRNA) in fetal intestinal epithelium ( P <.05) and NEC epithelium ( P <.01), by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), compared with the intestinal epithelium in children older than 1 year ( Fig. 1 A). Nuclear translocation of nuclear factor kappa B (NF-κB) also plays a role in the intestinal inflammatory response. Under the inactivated state, cytosolic NF-κB is bound to inhibitor of κB (IκB), which undergoes phosphorylation, ubiquitination, and proteasomal degradation when activated by a proinflammatory stimulus. The ultimate degradation of Iκβ allows nuclear translocation of NF-κB and subsequent transcription of proinflammatory cytokines. An exaggerated response of this pathway has been demonstrated in the immature intestine when exposed to colonizing bacteria and may be because of decreased expression of IκB in the resting state in immature human enterocytes (H4 cells) compared with mature enterocytes (T84 cells). Furthermore, intestinal toll-like receptor (TLR) activation is downregulated with advancing gestational age and may also contribute to the inflammatory responsiveness in the premature infant. Fig. 1 B and C show the balance of positive (eg, TLRs, NF-κB) and negative (eg, SIGIRR, Tollip) regulators, which promote a controlled inflammatory response. A significant increase in positive inflammatory regulator expression ( P <.01) and a decrease in negative inflammatory regulator expression ( P <.01) have been demonstrated in fetal intestinal epithelium, compared with mature intestinal epithelium. Further decreases in negative regulator expression were demonstrated in the intestinal epithelium from patients with NEC ( P <.001).
There are distinct functional characteristics of the preterm intestine, which are appropriate for intrauterine life and sterile amniotic fluid exposure but, however, inappropriate in managing the bacterial stimulation of extrauterine life. These characteristics include decreased peristaltic activity, increased intestinal permeability, immature bacterial adhesion site glycoconjugates, decreased secretory immunoglobulin A levels, deficient trefoil factor, defensin protein alterations, and aberrant intestinal mucous composition. The critical interplay between colonizing bacteria and the intestinal immune system is uniquely modified by the various immaturities of the intestinal immune system and several environmental factors encountered by the preterm infant. Abnormalities in this process likely contribute to the risk of NEC.
Host susceptibility in preterm infants
Immaturity of the innate intestinal immune system renders the premature infant who is poorly equipped to manage the immunologic challenges of extrauterine life. The immune system before delivery is T H 2-predominant and allows avoidance of maternal rejection of the fetus. The process of postnatal tolerance is dependent on a transition from T H 2-predominant to T H 1-predominant immunity and is influenced by the intestinal microbiome. The initial stages of bacterial colonization, occurring during the process of delivery and initiation of enteral nutrition, modify intestinal immunity and allow tolerance of further microbial stimulation. Specifically, the interaction between nutrients and early colonizing bacteria influences T-lymphocyte development and maturation. Recognition of luminal bacterial components by T-lymphocytes is critical to the balance between tolerance and development of gastrointestinal illness.
Maturation of intestinal innate immune responses occurs in a predictable manner in mature neonates; however, this process is poorly regulated in the setting of prematurity. As a result, the immature intestine shows an exaggerated inflammatory response after exposure to bacteria and bacterial products, compared with the mature intestine. Interleukin (IL) 8 is a chemokine, which has the ability to recruit neutrophils to areas of inflammation and therefore plays a critical role in the inflammatory response. Nanthakumar, and colleagues illustrated a significantly higher expression of IL-8 messenger RNA (mRNA) in fetal intestinal epithelium ( P <.05) and NEC epithelium ( P <.01), by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), compared with the intestinal epithelium in children older than 1 year ( Fig. 1 A). Nuclear translocation of nuclear factor kappa B (NF-κB) also plays a role in the intestinal inflammatory response. Under the inactivated state, cytosolic NF-κB is bound to inhibitor of κB (IκB), which undergoes phosphorylation, ubiquitination, and proteasomal degradation when activated by a proinflammatory stimulus. The ultimate degradation of Iκβ allows nuclear translocation of NF-κB and subsequent transcription of proinflammatory cytokines. An exaggerated response of this pathway has been demonstrated in the immature intestine when exposed to colonizing bacteria and may be because of decreased expression of IκB in the resting state in immature human enterocytes (H4 cells) compared with mature enterocytes (T84 cells). Furthermore, intestinal toll-like receptor (TLR) activation is downregulated with advancing gestational age and may also contribute to the inflammatory responsiveness in the premature infant. Fig. 1 B and C show the balance of positive (eg, TLRs, NF-κB) and negative (eg, SIGIRR, Tollip) regulators, which promote a controlled inflammatory response. A significant increase in positive inflammatory regulator expression ( P <.01) and a decrease in negative inflammatory regulator expression ( P <.01) have been demonstrated in fetal intestinal epithelium, compared with mature intestinal epithelium. Further decreases in negative regulator expression were demonstrated in the intestinal epithelium from patients with NEC ( P <.001).
There are distinct functional characteristics of the preterm intestine, which are appropriate for intrauterine life and sterile amniotic fluid exposure but, however, inappropriate in managing the bacterial stimulation of extrauterine life. These characteristics include decreased peristaltic activity, increased intestinal permeability, immature bacterial adhesion site glycoconjugates, decreased secretory immunoglobulin A levels, deficient trefoil factor, defensin protein alterations, and aberrant intestinal mucous composition. The critical interplay between colonizing bacteria and the intestinal immune system is uniquely modified by the various immaturities of the intestinal immune system and several environmental factors encountered by the preterm infant. Abnormalities in this process likely contribute to the risk of NEC.
Bacterial colonization of preterm infants
The sterile intestine at the time of birth is colonized by several bacterial strains and depends on early environmental exposures. This process is also affected by several host and microbial factors. The temporal patterns of bacterial acquisition and, ultimately, bacterial strain diversity are both relatively predictable depending on the gestational age at birth, mode of delivery, antibiotic exposure, and source of nutrition. Colonizing bacterial strains include Bifidobacterium , Lactobacillus , Escherichia coli and Bacteroides, Clostridia , Staphylococcus , and Pseudomonas aeruginosa. Vaginally delivered newborns are colonized earlier with beneficial strains, such as Bifidobacterium and Lactobacillus , and have smaller populations of Klebsiella , Enterobacter , and Clostridia , compared with those delivered via cesarean section. Although Bifidobacterium is the primary colonizing strain of breastfed infants, those receiving formula are primarily colonized with Bifidobacterium and Bacteroides. The intestinal microbiome shifts toward that of an adult after completion of weaning. The colonization process of preterm infants is further altered by multiple exposures encountered within the NICU environment, including broad-spectrum antibiotics, instrumentation, gastric acid blockade, and opioids, which compromise intestinal motility. A sophisticated relationship between the various colonizing bacterial strains is likely more critical to host health than the presence or absence of one particular strain. An optimal balance of bacterial diversity promotes intestinal health by enhancing immunity, decreasing gas production, inhibiting colonization by pathogenic bacteria, and improving nutrient metabolism and absorption.
Although the presence of luminal bacteria is required in the development of NEC, the disease process is considered as an inappropriate inflammatory response to bacterial components than a bacterial infection. Stool culture analyses during outbreaks of NEC have failed to identify a specific organism implicated in the pathogenesis of disease. Using these techniques, an association has been demonstrated with E.coli , Klebsiella pneumoniae , Clostridium , and Enterobacteriaceae. Sequencing analyses of stool had previously demonstrated a significant reduction of bacterial diversity and increased density of atypical strains in preterm infants who developed NEC, compared with healthy preterm infants. Using 16S ribosomal RNA sequencing techniques, Mai and colleagues compared the fecal microbiota of neonates with birth weight less than or equal to 1250 g and those younger than or at 32 weeks of gestational ages who developed NEC (Bell Stage ≥II) with matched controls. Stool samples were analyzed 1 week and 72 hours before the development of NEC. Although these data showed no statistically significant differences in bacterial variation at earlier time points in NEC infants or controls, a greater overall variation was noted in NEC infants 1 week before the development of NEC, compared with controls. Most of the samples showed a predominance of 4 phyla, Firmicutes, Proteobacteria, Bacteroidetes and Actinobacteria, with less Actinobacteria and Bacteroidetes in the NEC infants. Furthermore, although the 4 predominant phyla were stable between the 2 time points, Proteobacteria increased by 34% and Firmicutes decreased by 32% during the week before disease development in the NEC infants ( Fig. 2 ).






