Background
There were more than 67,000 new cases of urothelial carcinoma of the bladder and nearly 14,000 deaths from the disease in the United States in 2007 [1]. The majority of new cases of urothelial carcinoma of the bladder are diagnosed when the disease is not muscle invasive. However, 20–40% of new cases are muscle invasive at time of diagnosis and a significant proportion of patients diagnosed with noninvasive disease will eventually progress to muscle-invasive disease [2]. The current gold standard for treatment of muscle-invasive urothelial carcinoma is radical cystectomy with pelvic lymphadenectomy. In one large single-institution contemporary series of radical cystectomy outcomes, 5- and 10-year recurrence-free survival rates after surgery were 68% and 66% respectively. Overall 5- and 10-year survival rates in this series were 60% and 43% respectively, with 3% perioperative mortality [3]. However, the question remains: can we improve on these numbers with adjuvant/neo-adjuvant chemotherapy or perhaps spare the bladder altogether?
Clinical question 32.1
Does adjuvant chemotherapy after radical cystectomy for muscle-invasive urothelial carcinoma of the bladder confer a survival advantage?
Literature search
A systematic search was conducted using the PubMed database from 1990 to 2008 using the terms: “muscle invasive,” “urothelial carcinoma,” “radical cystectomy,” “transitional cell carcinoma,” “bladder cancer,” “neo-adjuvant” and “adjuvant chemotherapy.”
The evidence
There have been a total of 11 RCTs investigating adjuvant chemotherapy after radical cystectomy for bladder cancer, but only six of these trials had individual patient data available for subsequent review (Table 32.1). Two meta-analyses of these data have since been performed. All these studies compared cisplatin-based chemotherapy regimens with localized extirpative therapy alone (in the form of radical cystectomy). One of these trials investigated cisplatin therapy alone [4]; the other five used cisplatin-based combination therapy [5–9]. Individually, these trials enrolled a small number of patients, with total accrual ranging from 50 to 108 patients. Furthermore, interpreting and then subsequently utilizing these data in the clinic setting has been virtually impossible due to methodological flaws in their design and execution [10].
Table 32.1 Randomized controlled trials of adjuvant chemotherapy following radical cystectomy versus radical cystectomy alone
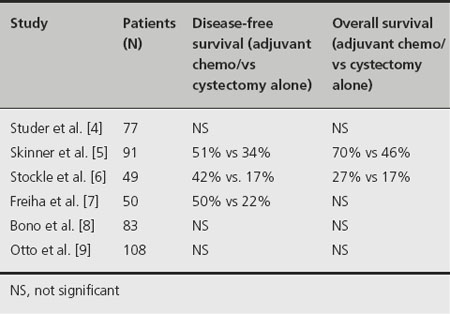
Inadequacies have been identified in each of the six aforementioned RCTs. These include major problems such as insufficient sample sizes, early cessation of patient entry, premature interim analyses, incorrect statistical methods, and failure to report important endpoints [11]. These shortcomings notwithstanding, a benefit of adjuvant chemo-therapy was demonstrated in three out of the six trials.
Skinner et al. demonstrated benefit for patients receiving adjuvant chemotherapy in time to progression, cancer-specific survival, and overall survival [5]. However, major criticisms of this trial include the fact that there was inadequate accrual of potentially eligible participants and therapy during the study was nonuniform. Moreover, the maximum benefit was observed in a subgroup that was not prospectively identified at the outset of the study [11].
Similarly, the study by Stockle et al. demonstrated increa-sed time to progression as well as improved cancer-specific and overall survival for patients who were randomized to adjuvant chemotherapy versus radical cystectomy alone [6]. Interestingly and importantly, patients in the cystectomy-alone arm were not offered the option of chemotherapy at time of recurrence, which may have affected the differences in survival.
Freiha and colleagues reported a benefit of adjuvant chemotherapy with regard to relapse-free survival but not overall survival [7]. Participants who recurred were offered the same chemotherapy regimen as patients who received adjuvant chemotherapy at the outset. The authors suggest that the lack of difference in overall survival may be attributable to the salvage of patients with metastatic disease who began chemotherapy only after recurrence was documented.
It should be emphasized that each of these three previously discussed studies that support the use of adjuvant chemotherapy was terminated early due to interim analyses that showed marked benefit in the chemotherapy arm [5–7]. A fourth RCT was stopped early after interim analysis revealed less benefit than anticipated [4]. Of note, the study by Studer et al. was the only trial that used cisplatin monotherapy [4]. Early cessation of RCTs is one of many recognized potential sources of bias and therefore calls into question the validity of the data reported in these studies [12].
In an attempt to address the many concerns about these individual studies and make use of the available data, two meta-analyses of these RCTs have been conducted [13,14]. The meta-analysis by the Advanced Bladder Cancer Meta-Analysis Collaboration (ABC) is the stronger of these two because it utilized individual patient data (IPD) analysis as opposed to pooled analysis. Therefore, the ABC meta-analysis will be the focus of the remainder of this discussion.
The ABC meta-analysis focused on the six previously mentioned RCTs. IPD was retrieved for a total of 493 patients. This represents 90% of all patients randomized in adjuvant chemotherapy trials comparing cisplatin combination therapy to cystectomy alone and 66% of patients randomized in all known bladder cancer adjuvant chemo-therapy trials. Follow-up data on two patients from the Bono et al. study were not available [8]. Therefore, final analysis consisted of 491 patients [13].
Overall survival and disease-free survival were the two primary outcome measures of this meta-analysis. There was a 25% relative decrease in the risk of death for patients treated with adjuvant chemotherapy (95% confidence interval (CI) 0.60–0.96). This equates to a 9% (95% CI 1–16%) absolute reduction in the risk of death for patients given chemotherapy at 3 years. However, further statistical analysis revealed that the study was underpowered to detect an absolute risk reduction for death below approximately 15% [13].
Disease-free survival was measured in 383 patients because data regarding recurrence were not available for one trial [9]. The reduction in relative risk of recurrence was 32% (95% CI 0.53–0.89) with an absolute risk reduction of 12% (95% CI 4–19%) at 3 years [13].
Comment
Proponents of adjuvant chemotherapy claim that patients with favorable surgical pathological findings at the time of radical cystectomy could be spared the toxicity and possible adverse effects of antineoplastic drugs. Indeed, subgroup analyses of both adjuvant and neo-adjuvant chemotherapy trials have revealed that patients with pT3–T4 disease are most likely to benefit from chemotherapy compared to patients with organ-confined disease. Furthermore, in studies of metastatic urothelial carcinoma, only 40–60% of tumors prove to be chemosensitive. Therefore, a counter-argument to the use of neo-adjuvant chemotherapy is that a significant percentage of patients who would not benefit from treatment (either because their disease would not need chemotherapy or they harbor tumor that is in fact resistant to chemotherapy) are not only being exposed to these potentially toxic drug regimens, but will also have potentially curative local therapy delayed. An adjuvant chemotherapy approach attempts to maximize individualization of treatment by assimilating the pathological findings at cystectomy.
There exist six RCTs investigating adjuvant chemotherapy after radical cystectomy for urothelial carcinoma of the bladder for which data were available for meta-analysis. Unfortunately, each of these trials has significant methodological flaws and the total number of patients is still small. A careful meta-analysis by the ABC group has yielded results that favor adjuvant chemotherapy after radical cystectomy. However, despite these encouraging numbers, the meta-analysis was underpowered to substantiate the reported survival benefits. More importantly, the underlying suboptimal quality of each individual study included in the meta-analysis makes it impossible to endorse the universal use of adjuvant chemotherapy in patients undergoing radical cystectomy for muscle-invasive bladder cancer. There are currently several large RCTs in progress attempting to answer this question. Treating physicians await the findings and should be encouraged to enroll patients in adjuvant chemotherapy trials.
Clinical question 32.2
Does neo-adjuvant chemotherapy for patients undergoing local treatment (either radical cystectomy or radiotherapy) for muscle-invasive bladder cancer result in a survival advantage?
Literature search
A systematic search was conducted using the PubMed database from 1990 to 2008 using the terms: “muscle-invasive,” “urothelial carcinoma,” “radical cystectomy,” “transitional cell carcinoma,” “bladder cancer,” “neo-adjuvant” and “adjuvant chemotherapy.”
The evidence
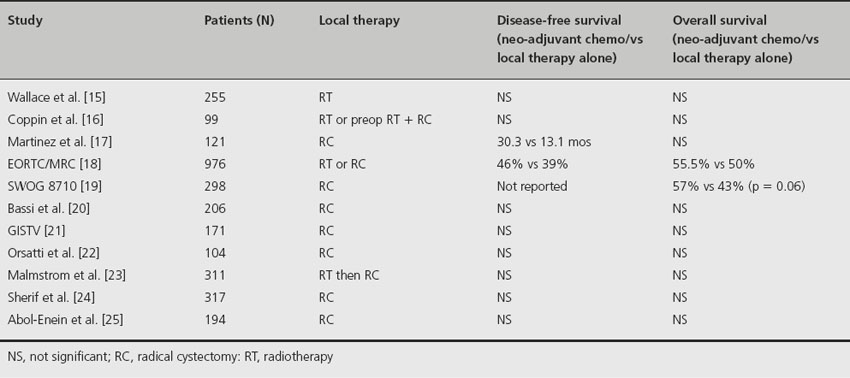
There are11 RCTs comparing neo-adjuvant chemotherapy to localized therapy alone (Table 32.2). Three of these trials involved cisplatin monotherapy and the others utilized cisplatin-based combination chemotherapy. Four of the trials included radiation therapy in some form as the means for local tumor control. Total patient accrual in each trial ranged from 99 to 976 patients. Individually, only the largest of the trials [18] demonstrated a significant overall and disease-specific survival benefit for patients receiving neo-adjuvant chemotherapy. In addition, two large meta-analyses of the data from these 11 RCTs have been performed and have confirmed a survival benefit for patients receiving neo-adjuvant chemotherapy for muscle-invasive bladder cancer prior to local treatment [26,27].
Table 32.2 Randomized controlled trials of neo-adjuvant chemotherapy prior to radiotherapy and/or radical cystectomy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








