Introduction
With 50,000 new cases and 13,000 deaths in the United States every year, kidney cancer represents a major oncological therapeutic challenge [1]. Approximately one-third of patients present with metastases at initial diagnosis and between 20% and 40% relapse after nephrectomy. Several clinical prognostic factors stratify patients with metastatic disease to low-, intermediate- and high-risk groups with median survival ranging from 22 months in the low-risk patients to 12 months in the intermediate- and 5 months in the high-risk groups respectively (Table 35.1). Only a small number of patients with metastatic kidney cancer can be cured by existing therapies. Renal cell carcinoma (RCC) is generally resistant to chemotherapy [2,3]. Due to a possible immunological influence in well-documented cases of spontaneous regression of RCC metastases, attention has focused historically on the possible means of modifying biological response in these patients. In the 1980s and 1990s the immunocytokines interleukin-2 (IL-2) and interferon-alpha (IFN-alpha) became the standard systemic therapy for metastatic renal cell carcinoma based on documented durable responses in some patients.
Table 35.1 Memorial Sloan Kettering Cancer Center (MSKCC) risk factor stratification scale
WHO, World Health Organization
| Factor | Poor prognosis |
| Karnofsky Performance Score | < 80 (WHO > 1) |
| Time from diagnosis to treatment | < 12 months |
| Hemoglobin | Below normal range |
| Lactate dehydrogenase | > 1.5 upper limit of normal |
| Calcium | > 10 mg/dL |
| Good risk | 0 prognostic factors |
| Intermediate risk | 1–2 prognostic factors |
| Poor risk | > 2 factors |
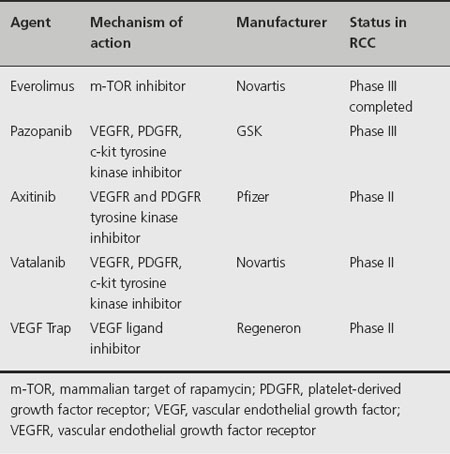
Recent discoveries and better understanding of signaling pathways and targets important in the development and progression of renal cell carcinoma have resulted in significant expansion of therapeutic options of patients affected with this disease. Sunitinib malate, sorafenib tosylate, bevacizumab ± interferon-alpha, temsirolimus and ever-olimus have improved clinical outcomes in randomized clinical trials by inhibiting the vascular endothelial growth factor (VEGF), m-TOR and related pathways. Many other targeted agents are in clinical development, representing the main focus of clinical research in RCC (Table 35.2). The rapidly changing therapeutic landscape of metastatic RCC raises several important questions related to the appropriate selection and sequence of available agents, the role (if any) of immunotherapy and the relevance of surgical debulking of these patients.
Table 35.2 Agents in development for treatment of renal cell carcinoma
Clinical question 35.1
Is there evidence that debulking nephrectomy provides clinical benefit in asymptomatic patients with metastatic renal cell carcinoma?
Background
Historically, the hypothesis that cytoreductive nephrectomy may play a role in therapy of metastatic kidney cancer was based on observation of occasional “spontaneous” regressions of metastatic tumors after nephrectomy. Other possible advantages to debulking nephrectomy include prevention of complications during subsequent treatment and improving patient performance status by elimination of the source of additional metastases, hemorrhage, and discomfort. However, nephrectomy performed as a single treatment modality for metastatic disease does not appear to have significant effect on survival [4]. The renewed interest in debulking nephrectomy came to light in the context of immunotherapy for metastatic RCC and the hypothesis that persistence of a large primary tumor may generate immunosuppressive cytokines and negate the therapeutic benefits of immunostimulatory agents. More recently, the introduction of agents with more potent systemic activity against RCC led, yet again, to the re-emergence of the question of contribution of debulking nephrectomy to patient outcomes in the new era of targeted therapies.
Literature search
Potentially relevant studies were identified by a computerized search, restricted to English-language literature, of the Medline electronic database (source PubMed, 1966 to March 2009) using relevant text and keywords in combination as follows: “metastatic renal cancer and nephrectomy,” “cytoreductive nephrectomy,” “debulking nephrectomy.” The reference lists of retrieved eligible articles were reviewed to identify additional relevant articles.
The evidence
Two prospective randomized clinical trials in patients with ECOG performance status of 0–1, Southwest Oncology Group (SWOG) 8949 [5] and European Organization for Research and Treatment of Cancer (EORTC) 30947 [6], reported statistically significant survival advantage for the combination of nephrectomy and IFN-alpha. Pooled analysis of these two trials demonstrated a median survival of 13.6 months for the combined arm versus 7.8 months for IFN-alpha alone which represented a 31% reduction in the risk of death (p = 0.002) in patients treated with nephrectomy and IFN-alpha [7]. Cytoreductive nephrectomy improved overall survival independently of patient performance status (0 or 1) and the site of metastases.
Comment
These two trials established debulking nephrectomy as a standard of care for patients who were considered candidates for systemic immunotherapy. The advent of antiangiogenic and targeted agents, as a preferred systemic therapy for metastatic RCC, raises several questions regarding the validity of the paradigm of cytoreductive nephrectomy as the vital component of therapeutic strategy for metastatic kidney cancer. It is uncertain whether the biological factors responsible for enhanced effects of immunotherapeutic agents after debulking surgery remain relevant in conjunction with antiangiogenic treatments. There are no prospective randomized trials addressing this issue but it is important to stress that the vast majority (> 90%) of patients treated on pivotal trials that established beneficial effect of antiangiogenic therapies in metastatic kidney cancer underwent prior cytoreductive nephrectomy.
Recommendations
A randomized clinical trial comparing the effectiveness of new targeted agents with or without debulking nephrectomy would address this valid scientific question but at this point it is not certain if such a study will be conducted. Until evidence to the contrary is established, it is prudent to incorporate cytoreductive surgery for all patients who are appropriate surgical candidates for that procedure. This represents a conditional recommendation for cytoreductive nephrectomy based on low-quality evidence according to Grade.
Clinical question 35.2
With the advent of targeted therapies in renal cell carcinoma, what are the clinical circumstances in which immunotherapy with high-dose IL-2 may be considered as the initial therapeutic option?
Background
Interleukin-2 is a cytokine which activates T cells and natural killer cells, stimulating them to produce a variety of other cytokines, such as IFN-gamma, granulocyte-macrophage colony stimulating factor, and tumor necrosis factor alpha, which in turn stimulate the activity of other cells in the immune system, such as the monocyte-macrophage lineage. It was first administered to patients with advanced RCC in the early 1980s. The initial reports of the efficacy of high-dose IL-2 (HDIL-2) in RCC demonstrated that a small fraction of patients achieved durable complete responses. Based on these results, the Food and Drug Administration (FDA) approved HDIL-2 therapy for advanced RCC in 1992.
Literature search
Potentially relevant studies were identified by a computerized search, restricted to English-language literature, of the Medline electronic database (source PubMed, 1966 to March 2009) using relevant text and keywords in combination as follows: “metastatic renal cancer and interleukin-2,” “metastatic renal cancer and high-dose interleukin 2,” “immunotherapy and kidney cancer.” The reference lists of retrieved eligible articles were reviewed to identify additional relevant articles
The evidence
Consecutive series of patients treated with HDIL-2 in the Surgery Branch of the National Cancer Institute from September 1985 through December 1992 were reported by Rosenberg et al. in 1994 [8]. Two hundred and eighty-three patients with metastatic melanoma or metastatic renal cell cancer who had failed standard treatment for their cancers received IL-2 at a dose of 720,000 IU/kg intravenously every 8 hours for a maximum of 15 doses per cycle. Ten patients (7%) with metastatic renal cell cancer experienced complete regression and 20 patients (13%) had partial regression; 78% of patients with complete regression have remained in complete remission from 7 to 91 months after treatment. The response rates of these initial studies have been subsequently updated, demonstrating that some patients with complete responses have not relapsed and may be effectively cured [9].
Comment
High-dose IL-2 remains the only potentially curative treatment in selected patients with metastatic RCC. Unfor-tunately, HDIL-2 treatment is associated with significant toxicity requiring ICU monitoring. Capillary leakage is almost universal and leads to hypotension, renal toxicity, altered mental status, fluid shifts, pulmonary edema, myocardial infarction, thrombocytopenia, infection, and mortality of 1–2%. Attempts to decrease the toxicity of HDIL-2 have so far been unsuccessful. Lowering the dose appears to reduce the effectiveness of therapy. A randomized clinical trial comparing HDIL-2 and low-dose IL-2 (LDIL-2) demonstrated no significant differences in overall and progression-free survival for the whole study population, but only HDIL-2 therapy resulted in durable complete responses in a small fraction of patients [10].
Recommendations
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








