Reference
Protocol
Success of pregnancy
Shulman et al. [50]
Male patients receive 96 mg/day methylprednisolone from days 21 to 28 of the female menstrual cycle
44 % pregnancy rate in first 12 months
Hendry et al. [31]
Male patients receive 40 mg/day methylprednisolone on day 1 of the female menstrual cycle followed by 5 mg/day from days 11 to 12
33 % pregnancy rate/treatment cycle
De Almeida et al. [33]
Male patients receive 2 mg/day of dexamethasone for a total of 13 weeks
Success measured by reduction of ASA titers (rate of reduction is between 0 and 50 %)
Haas et al. [32]
96 mg/day methylprednisolone in three divided doses for 7 days followed by a 2-day tapering of the drug, repeated for three cycles
Decrease of sperm-associated IgG
Hendry et al. [34]
Male patients receive 20 mg twice daily on days 1–10 of the female partner’s menstrual cycle, followed by 5 mg on days 11 and 12
31 % pregnancy rate in 9 months treatment cycle
Improvement in the pregnancy rates has been reported after GCSs therapy [30, 31]. Studies have reported between 0 and 40 % pregnancy rates after treatment. In a prospective, double-blind, placebo-controlled study of 43 men given three repeated monthly regiments of 96 mg methylprednisolone in three divided doses for 7 days followed by a 2-day tapering of the drug, corticosteroids did not have any effect on semen parameters, sperm-associated IgA, and plasma IgG ASA, or pregnancy rate. However, corticosteroids significantly reduced sperm-associated IgG, with no translation into any clinical benefit [32]. Similarly, other studies that looked at the effect of prednisone showed no significant effect on fertility, serum antibody levels, semen parameters, and sperm characteristics, but a slight decrease in the titer of seminal antibodies was observed [33]. Other protocols showed slightly favorable outcomes. In a cohort of subfertile men that were enrolled in a double-blinded crossover trial with circulating antibodies to spermatozoa who received prednisolone (20 mg twice daily on days 1–10 of the female partner’s menstrual cycle, followed by 5 mg on days 11 and 12) for 9 months resulted in a pregnancy rate of 31 % in the treatment group compared with a 9 % in the untreated group. However, there were no changes in the semen parameters in this cohort of patients [34]. The response to GCSs treatment is more likely in men with persistent level of ASA than in men with an acute autoimmunological response. Infertile men with ASA detectable for more than 1 year and treated with steroid responded effectively in suppressing all isotypes of ASA, and improved sperm motility. Further, the presence of high titers of isotype IgG against the tails of spermatozoa, and sperms with type I motility were predictors of pregnancy [35]. Comparing GCSs to other treatment modalities in a crossover randomized study, the effectiveness of intrauterine insemination (IUI) was significantly better than timed intercourse with cyclic low dose (20 mg) prednisolone therapy. The pregnancy rate before crossover for the IUI group was 16.7 %, whereas no pregnancies occurred in the steroid-treated group.
The benefit of steroid treatment, if any, must be judged against the potential adverse effects. Steroid therapy may cause several side effects, such as acne, dyspepsia, skin rashes, fluid retention, and mood changes [36]. The potential adverse effects and the lack of effectiveness in many cases have decreased the enthusiasm for steroid use. Interest in the use of other immunosuppressing agents such cyclosporine in autoimmune orchitis showed some promising results [37]. In men with ASA treated with cyclosporin A (5–10 mg/kg/day) for 6 months the serum ASA fell in 33 % of the subjects on treatment, and sperm count and motility increased substantially in some [38]. However, this was a very small study with no placebo controls and conclusions cannot be drawn.
Laboratory Techniques and Sperm Washing
Sperm preparation , in subjects with ASA, as a preparation for artificial insemination, was first tried by Halim [39]. Since then, multiple techniques for sperm preparation and washing have evolved. The concept of removing ASA from semen or ASA bound to sperm can be classified into three categories: (1) preventing the binding of ASA to sperms, (2) removing bound ASA from sperm surface by immunobead exchange or by using proteases that will cleave the immunoglobulin, and (3) separating ASA-coated sperms from noncoated sperms. Even though some of these techniques are promising, conflicting outcomes, and the technical challenges needed to master some of these techniques have prevented them from wide utilization in clinical practice.
Preventing ASA Sperm Binding
In the effort to prevent the binding of ASA to the sperm, multiple techniques have been employed. In the past, it was thought antibodies are secreted from the prostate and seminal vesicle, and that ASA bind to sperm during or after ejaculation [40]. To stop the ASA binding to sperm, semen is collected into a medium containing 50 % heterologous serum or albumin, followed by rapid dilution or washing to increase the proportion of antibody-free spermatozoa in the specimen. Studies have suggested that combining this technique with IVF increased the rate of fertilization, but it is not clear if the success is due to the decrease in the ASA binding to sperm or to selecting good sperm in the washing process [41]. However, subsequent analysis have proven that rapid dilution or washing to be ineffective in collecting sperm that is free of bound ASA [42]. The efficacy of rapid dilution of semen on sperm-bound spermatozoa followed by swim up in HAM F-10 and 10 % human serum was evaluated, and it was found that the diluting semen after ejaculation did not significantly change sperm-bound antibodies detected [43]. To the contrary, antibody secretion and biodistribution studies in the male reproductive tract using 125I-labeled anti-FA-1 IgG in mice revealed that the antibodies are preferentially secreted in epididymis and vas deferens to bind to sperm cells. These findings indicate that antibodies bind to sperm before ejaculation not after ejaculation as it was initially thought [44]. Hence, rapid washing and dilution of the ejaculate will not affect the binding of the antibodies to the sperm.
Eliminating Bound ASA from Sperm Surface
The use of immunobead incubation to treat ASA-positive sperm has been described. Assessment of sperm incubation with immunobeads showed that both serum and semen immunoglobulin binding to sperm decrease over time. It was thought that sperm-covered ASA and immunobead coincubation results in a decrease in the number of sperm bound to ASA, improving outcome [45, 46]. The use of immunobead adsorption itself seems striking for the removal of ASA off the spermatozoa, but the efficacy is not completely clear. Studies that used lyophilized immunobead adsorption concluded that this technique can be applied in the selection of antibody-free spermatozoa, and the rate of recovery of spermatozoa decreases as the number of antibodies bound to sperm increases [47]. The success of immunobead incubation in the treatment of immunologic infertility seems to be limited with no consistent benefits observed. According to the published literature, there are still plenty of questions that need to be addressed regarding the effect of this technique on the sperm membrane. Despite the limitation of the immunobead test in treating men with ASA, the information provided by the test results when it is performed is a very important prognostic tool. The immunobead test can help to identify the immunoglobulin subtype and the location of the antibody on the sperm [48]. The test is widely available, simple to run, and provides titers that are useful in monitoring patients who are being treated for ASA (i.e., corticosteroid treatment).
Simple centrifugation and washing of the sperm was first reported by Hanson et al. [49]. It is a very simple technique that is utilized in sperm preparation for IUI. Fresh seminal fluid from men with ASA is centrifuged and suspended in an albumin solution or nutrient medium before using it for insemination [50]. The effectiveness of sperm washing is dependent on the time of ejaculation to washing, limited to the IgG subtype, and it is less likely to yield motile sperms when compared to the swim-up method of preparation [51]. The use of centrifugation and simple wash procedure in men with immunological infertility has multiple drawbacks. Cell debris, white blood cells, bacteria, and sometime free ASA are part of the pellet and they are capable of causing irreversible damage to the viable sperms [52]. One way to filter the sperm from other elements is to use the ability of the sperm to swim. Both the swim-up and swim-down methods can be used to separate motile sperms up or down, respectively, in a nutrient rich medium. The efficiency of these techniques depends on the quality of the original specimen, and the quantity of sperm and ASA. Antibodies on the sperm surface cause agglutinated and poorly progressive spermatozoa, and therefore this results in limited recovery of sperm using the swim-up or swim-down methods [53]. This limitation makes these methods of sperm preparation poor treatment options for patient with ASA.
Enzymatic cleavage of immunoglobulin has also been proposed as a treatment option for men with ASA. IgA proteases derived from Neisseria gonorrhoeae, whose substrate specificity is limited to Fc region of human IgA type 1, have the ability to cleave IgA and reduce the total number bound to sperms with improvement in sperm cervical mucus penetration ability [54, 55]. However, IgA proteases are very specific and it is not clear how effective they are in clearing other antibody isotopes. In addition, since the site of the cleavage is in the Fc region, part of the immunoglobulin remains attached to the sperm. Since IgM, IgG, and IgA2 are not cleaved with IgA proteases, other proteases have been used with some success. It have been previously demonstrated that the three enzymes, trypsin (500 U/ml), chymotrypsin (500 U/ml), and papain (50 U/ml), are capable of decreasing immunologically agglutinated spermatozoa with no detrimental effect on sperm motility or vitality [56]. The efficacy of these enzymes and possible negative effect on sperm are not completely understood. Post-treatment testing showed some mixed results, the incubation of normal spermatozoa with either chymotrypsin or papain resulted in impairment of oocyte penetration in the zona-free hamster egg penetration test. The use of trypsin, while significantly improving oocyte penetration of spermatozoa had no effect on the sperm mucus interaction [57].
Magnetic isolation of antibody-coated sperm from antibody-free sperm to avoid potential damage to fragile sperm through centrifugation has been tried. Superparamagnetic polymer microspheres coated with monoclonal antibodies are used to isolate antibody labeled from antibody-free spermatozoa (see Fig. 23.1). Using magnetic isolation, viable spermatozoa are isolated, but the motility of the isolated spermatozoa deteriorated rapidly [58]. ICSI would, therefore, be necessary for fertilization with the antibody-free sorted sample.
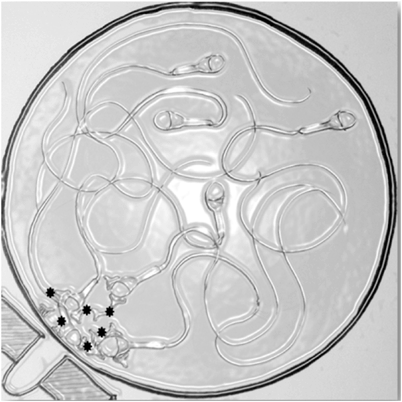
Fig. 23.1
Magnetic separation of antibody-labeled from antibody-free spermatozoa, a magnet separates ASA-bound spermatozoa by attraction of magnetic beads; only ASA-free sperm last in the supernatant
The different techniques that attempted to select sperm with no ASA showed some success, but most of the published studies are small series with no randomized control trials [47]. Although sperm preparation techniques are capable of separating ASA-free sperm, they still require the utilization of intrauterine IUI, or ART-like ICSI to achieve pregnancy. Furthermore, the consequence of these techniques on sperm is not completely understood.
Assisted Reproductive Technologies
There is a growing body of knowledge about interrelationship between ASA and ART. Although ART may be used to overcome ASA-related infertility, ASA may have a detrimental effect on ART outcome. Several studies have examined the use of IUI, gamete intrafallopian transfer (GIFT), IVF , and ICSI procedures for the treatment of immune infertility.
The results of the technique for ASA detection must be taken into account with regard to the selection of the best assisted reproductive technique. If sperm was found on a postcoital cervical mucus test, then IUI, to bypass the mucus, might be a good and inexpensive option. In the patient that has antibodies to the head of sperm, ICSI should be considered. Two factors need to be taken into account in presence of ASA when considering ART. First, ASA interfere with sperm passage through the female genital tract or with egg fertilization. ART itself may lead to ASA production in the female partner after sperm introduction (i.e., IUI). When offering couples ART, it is important to be aware of the female ASA titers. The presence of ASA in the female partner has multiple implications; IUI in the presence of ASA has the potential to be secreted in the female genital tract and impedes the sperm progressive motility. In such circumstance, it is reasonable to skip artificial insemination and to proceed directly to IVF [59]. Oocytes from females with positive ASA titers should be washed very carefully from follicular fluid since they will also be positive for ASA. Therefore, the evaluation of the presence of ASA in the serum of women involved in IVF treatment should be performed to avoid unexplained failure and unnecessary treatments.
Intrauterine Insemination
The use of various techniques as were discussed earlier to obtain ASA-free sperms has been very unpromising. Therefore, the recovery of motile antibody-free sperm makes the use of IUI limited to certain subsets of patients. One of the causes of immune-mediated infertility is the inability of sperm to penetrate the cervical mucus . Therefore, the rationale for IUI in this situation is to place the sperm beyond the cervix [60]. (See Fig. 23.2) There is a lack of controlled, prospective studies evaluating the outcomes in the patient with immune infertility being treated with IUI. When women who are positive for ASA in the cervical mucus are partnered with a male partner who is ASA negative, the pregnancy rate after IUI was identical to women who did not have ASA [61]. These findings were not consistently seen in other studies. Nevertheless, in the setting of controlled ovarian stimulation , IUI significantly improved pregnancy rates [62, 63]. In the setting of men with documented ASA, investigators have shown sperm washing before IUI might have little or no effect on improving the probability of pregnancy with some investigators reporting a 0 % success rate [64–66]. There is no good evidence that IUI alone or in combination with specific-sperm preparation techniques might help ASA-positive infertile men. However, some studies have reported a good pregnancy rate after IUI in ASA-positive infertile men with a poor postcoital test compared to IUI in ASA-negative infertile men [67] with ASA-positive men achieving near 33 % pregnancy rate with IUI compared to 19 % in IUI couples for other reasons [68].
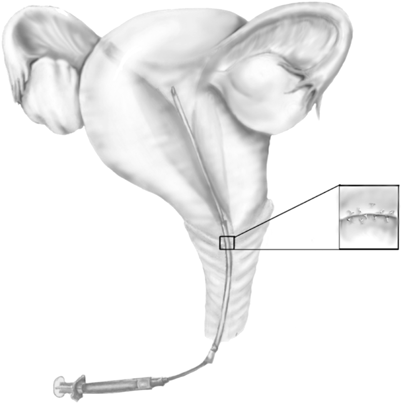
Fig. 23.2
IUI to bypass cervical mucus ASA
In Vitro Fertilization
IVF requires low number of sperms (100,000) to fertilize retrieved oocytes, and only enough viable sperms are needed as mature oocytes, making it useful to treat couples with male factor infertility. Studies on fertilization rates following IVF have reported contradictory results in couples identified as having ASA. Some studies demonstrated a detrimental effect of ASA on fertilization and pregnancy rates and others did not. However, comparing such studies is a challenge because of the heterogeneity in study design, ASA test type, and the wide range of ASA cutoff levels that are used (Table 23.2). Several studies have shown that IVF used to treat patients with suspected immunological factors have lower pregnancy rate than when it is used for other etiologies, with an inverse relationship between ASA titers and fertilization rates [69–73]. In men with > 80 % immunoglobulin-bound sperm, the fertilization and pregnancy rate was as low as 27 and 0 %, respectively. When < 80 % was bound, the fertilization and pregnancy rate was 72 and 67 % [74]. This correlation and the effect of ASA titers on fertilization were confirmed in other studies [75]. In contrast, there are studies that found fertilization to be identical in ASA-positive and ASA-negative populations [76], and these finding were corroborated by a systematic review of 16 studies involving 4209 ART treatment cycles (1508 IVF and 2701 ICSI cycles). The meta-analysis concluded that semen ASA status was not associated with pregnancy rates in IVF and ICSI, and the mean pregnancy rates for the IVF and ICSI groups were 23 and 36 %, respectively. This extensive meta-analysis suggests that both standard insemination with IVF and ICSI are viable options for infertile couples with semen ASA [77] .
Table 23.2
ART and pregnancy rates in men with antisperm antibodies (ASA), immunobead binding test (IBT), and mixed antiglobulin reaction (MAR)
Study | Total ART cycles | Total cycles with ASA | ART procedure | Assay used for detection | Percent pregnancy |
|---|---|---|---|---|---|
Lahteenmaki [75] | 156 | 47 | IVF | IBT and MAR | 24 |
Acosta et al. [71] | 67 | 38 | IVF | IBT | 39 |
Vujisic et al. [72] | 52 | 14 | IVF | IBT | 29 |
Clarke [73] | 89 | 38 | IVF | IBT | 8 |
Nagy et al. [83] | 1822 | 54 | ICSI | MAR | 28 |
Clarke et al. [80] | 179 | 39 | ICSI | IBT | 13 |
Check et al. [81] | 93 | 26 | ICSI | Not reported | 42 |
Esteves et al. [82] | 351 | 49 | ICSI | IBT | 53 |
The prognosis of IVF in couples with male ASA as a cause of infertility might dependent on the class and physical location of the antibodies on the sperm. In a retrospective study that evaluated the impact of immunoglobulin isotype and location of binding in 48 ASA-positive couples undergoing IVF, they noted that IgA significantly reduced fertilization rates only when it was associated with IgM and was present on the sperm head. The presence of IgM either on the sperm head or end of the tail also adversely affected fertilization rates [78]. Despite the reported success of IVF in these populations, it is important to note that the quality of embryos obtained after IVF using sperm from ASA-positive men is generally poorer when compared to embryos from ASA-negative men [24]. Unexpected fertilization failure happens rarely when the male has normal semen parameters. This can be due to an oocyte defect or a sperm defect and certainly ASA have been implicated in complete failure of fertilization despite normal semen parameters. The discussion with the couple undergoing treatment about this possibility should be part of every IVF consent consultation .
Intracytoplasmic Sperm Injection
IVF and ICSI have become a routine and widely acceptable procedure to treat infertility. In the ICSI procedure, a single sperm is injected into the cytoplasm of the oocyte. When immune infertility is not overcome by standard IVF despite normal fertilization rates, ICSI should also be considered [79–82]. ICSI has the potential to overcome antibody-mediated infertility that interferes with the interaction between the sperm and the oocyte’s ZP and oolemna. In a study looking at 29 infertile ASA-positive couples treated with ICSI after failing fertilization with IVF, the fertilization and cleavage rates were not significantly different between ASA-positive and ASA-negative groups: 79 vs. 89 % [24]. A retrospective analysis of 55 ICSI cycles for 32 different couples with greater than 80 % of ASA-bound sperm demonstrated no difference in pregnancy rate (30 %) between the ASA-positive and ASA-negative groups undergoing ICSI [83]. Generally, there are no relationships between ASA levels and pregnancy rates or fertilization rate, and the use of ICSI leads to a better prognosis in couples where the man is ASA positive [77]. Despite the relatively good prognosis , care should be taken when counseling patients with ASA infertility since there is some evidence that ASA can have postfertilization effects on the preimplantation and developing embryo [84].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





