Fig. 12.1
Proctoscope held in place by Martin arm
Several authors extol the benefits of the combined multifunctional endosurgical unit which automatically regulates suction, irrigation, and gas insufflation to maintain a constant intrarectal pressure of 12–15 cm H2O [6]. Maintaining constant intrarectal pressure is essential, as this facilitates visibility of the operative field. If the rectum does not distend properly, or if the system does not maintain a constant intrarectal pressure, this is a sign that there is an air leak in the system. This is the most frequently encountered problem that the TEM surgeon must learn to troubleshoot as loss of pneumorectum results in complete loss of surgical view [6]. The constant insufflation from the TEM unit avoids the billowing effect seen when distending the rectum with a laparoscopic insufflator.
Instruments
In order to permit mobility within the 4 cm operating proctoscope, a unique design element has been built in to each of the TEM instruments, notably that they are all angled instruments. A slight bend in the instrument shaft near the working end allows for maximal ergonomic positioning and function within the closed operating field [7]. The need for intracorporeal knot tying is circumvented by the use of a clip applicator which applies a silver clip to each end of the suture material. Several other innovative instruments are available to improve the TEM operating experience, particularly the multifunctional TEM 400 instrument by Erbe (ERBE Elektromedizin GmbH, Tubingen, Germany). Many surgeons use the 5-mm ultrasonic harmonic scalpel which facilitates hemostatic dissection particularly when traversing the mesorectum; however, we favor the angled cautery device [7]. Unfortunately, the multifunctional ERBE instrument which allows for suction, irrigation, and cautery in one instrument is not currently available in the United States (Fig. 12.2).
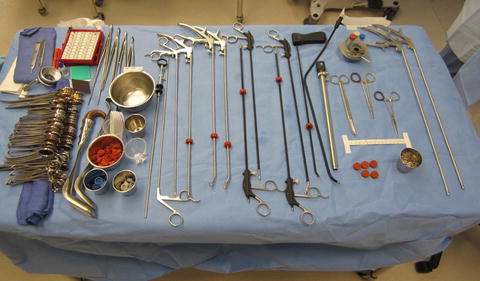

Fig. 12.2
TEM surgical instrument table
Patient Positioning
In order to facilitate efficient surgical resection, patient positioning is paramount. There are several principles which must be applied to TEM positioning to allow for optimal surgical visibility: the bevel of the scope must face down at the lesion, and the lesion must be maintained at the center of the operating rectoscope throughout the dissection. Because the operation is performed through a 20 cm long operating rectoscope there is very little movement of the instruments either up/down or left/right. The lens system occupies the upper 180° of the rectoscope, therefore it is imperative that the lesion is in the bottom half of the scope. Scheduling should make mention of patient position so that all team members are aware. Also of note, full-thickness excision is historically felt to be possible extraperitoneally up to the 20 cm level posteriorly, the 12 cm level anteriorly, and the 15 cm level at each respective side wall [7]. Particular attention must be paid to positioning as it has been referred to as the most important aspect of this operation [6]. Higher lesions can be approached by experienced TEM surgeons, but a lesion where entry into the peritoneum is possible should be avoided by surgeons early in their TEM learning curve (Fig. 12.3).
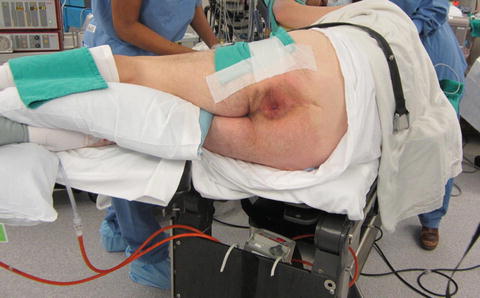

Fig. 12.3
Patient in right lateral position prior to draping
Personnel Positioning
The surgeon will be seated between the patient’s legs, the operating table and surgeon’s stool can be adjusted to promote a comfortable operating environment. The accessory scope must be inserted and imaging routed to the external monitor to allow for visualization by residents, medical students, and operating room staff. The multifunctional endosurgical unit will be positioned close to the operating field where the tubing can safely reach the field. The scrub nurse should be positioned opposite the endosurgical unit, and the surgical assistant should be seated next to the surgeon (Fig. 12.4).
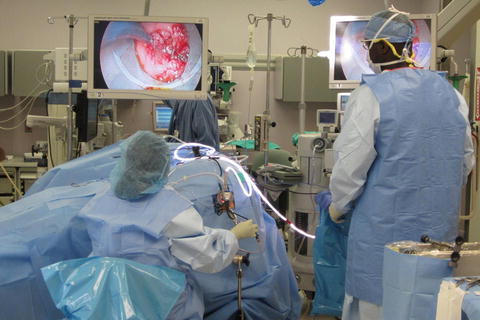

Fig. 12.4
Personnel positioning during TEM with patient in right lateral position
Operative Technique
As previously discussed, positioning is of the utmost importance as the lesion is optimally in the bottom 180° of the scope to allow adequate reach. Correspondingly, posterior lesions are best managed with modified lithotomy, anterior lesions with prone positioning, left-sided lesions necessitate left side down, and right-sided lesions must be addressed with right side down. After the patient is prepped and draped, the anus is gently dilated, and the 4 cm operating proctoscope inserted. The scope must then be manipulated until an ideal placement of the lesion is identified in the lower half of the operating proctoscope. To do this a glass faceplate is employed and the scope is manipulated similar to a large rigid sigmoidoscope.
Once the desired visual field has been obtained, the operating proctoscope is secured to the table via the Martin arm, and the operating faceplate is closed on the end of the scope. The rubber sleeves and caps are placed onto the working ports, and must be kept lubricated with mineral oil as drying and cracking of the caps are a possible cause of intraoperative air leak with subsequent loss of pneumodistention.
Attention must now be turned to the set up of the multifunctional endosurgical unit. There are four important pieces of tubing associated with this apparatus which provide continuous insufflation, regulation of intrarectal pressure, irrigation, and roller-pump suction. All of the attachments are suited only for the proper tubing connectors which avoids possible connection mistakes.
After proper set up of the operative field, the lesion is infiltrated using local anesthetic with epinephrine to aid in hemostasis. At this point in time the margin of the lesion is marked out using electrocautery (Fig. 12.5). A 5 mm margin is ideal for adenomas, and 10 mm for carcinomas which are amenable to TEM resection. It is important to perform this step now as it is often hard to be certain of margins once cautery artifact, blood, and smoke obscure the operative field. Additionally by performing this, the surgeon confirms his/her ability to reach all margins of the lesion.
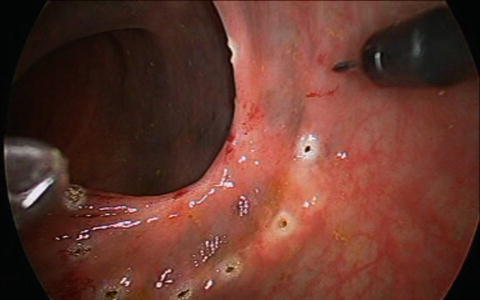

Fig. 12.5
Margin of lesion marked with electrocautery
Full-thickness excision is required for all malignant lesions, while adenomas can be excised in either a full-thickness or submucosal plane (Fig. 12.6). Dissection is carried out using electrocautery. Care must be taken when addressing anterior lesions, as the peritoneal reflection may be present at varying levels. Entry into the peritoneal cavity used to be an absolute contraindication for this approach. It is still advisable to avoid high anterior lesions early in one’s TEM experience. As experience has grown we recognize that this can be safely managed transanally. The possibility of the need for an abdominal operation and even proximal diversion must be discussed preoperatively with every patient. This should be an exceedingly rare event. In the event of this rare complication, expectant management is a key tenet as patients can potentially develop intra-abdominal sepsis requiring reoperation. After successful dissection, all wounds should be irrigated and subsequently closed with a running transverse suture (Fig. 12.7).
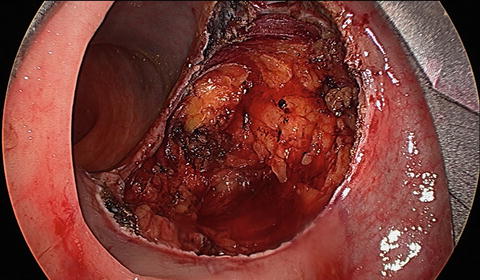
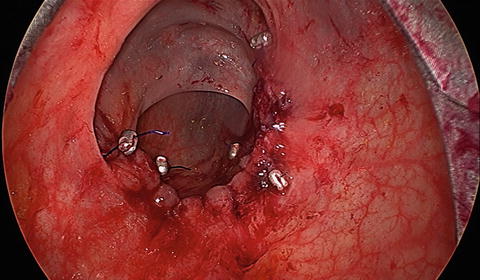

Fig. 12.6
Full-thickness local excision

Fig. 12.7
TEM running anastomosis
Technical Pearls
Patient positioning is very important and can be referred to as the most important principle of TEM. Remembering to reposition the operating rectoscope is of the utmost importance. This allows the TEM resection to proceed in the most efficient fashion by continuing to keep the lesion in the bottom of the scope. The TEM proctoscope must be entirely encompassed by the anus to allow for adequate pneumorectum, which can be difficult to achieve below the 5 cm level. Lesions which are located in the very distal rectum (below the 5 cm level) may be better addressed with conventional local excision. Many experienced TEM surgeons have become so accustomed to the superior view afforded by this technology that they prefer to use the TEM scope. To facilitate this more complicated dissection, one must change from a bevelled to a flat operating scope. In this situation, the closure is often done in a conventional transanal fashion.
Air leak is by far the most frustrating situation encountered by the TEM surgeon. Air leak leads to loss of pneumorectum and potentially complete collapse of the operative field. When encountered it is important to evaluate an air leak in a systematic fashion. Trouble shooting the dreaded airleak involves checking all equipment tubing and connections, as well as the rubber caps which are present on the faceplate for any cracks or pinholes.
There are several key pieces of information which must be kept in mind when manipulating the TEM instruments. First, the instruments should always remain in parallel, and should not cross over each other. There is very little movement in the horizontal axis as one is operating through a 20 cm tube. To gain mobility the instruments are moved in and out and rotated slightly, utilizing the angle of the instrument to gain reach and mobility. Instruments should be lubricated with mineral oil to reduce wear and tear on the face plate caps which can lead to loss of operative visibility and to reduce the annoyance of drag on your instrument as you are trying to dissect. The suction catheter should be maintained in the proximal neck of the rectoscope, well out of the operative field of view, and away from the rectal wall. By keeping the suction out of the operating field, the surgeon increases the mobility of the working instruments.
When suturing the defect closed, the surgeon must carefully pass the needle from hand to hand. Dropping the suture needle may necessitate repositioning of the rectoscope, and will subsequently lead to delay in progression of the operation. The suture material must be kept short in length or a significant amount of time will be required to simply pull the suture material through the tissue. Generally we recommend a 10 cm stitch length. If the surgical resection margins necessitate an extensive resection, the large defect which ensues must be closed with multiple sutures. Fortunately even after closure of a large defect, the patency of the rectal lumen is assured as the surgeon has been closely monitoring it through the 4 cm operating proctoscope for the duration of the closure.
Indications
Benign Disease
The benefit of TEM is obvious when radical surgery can be avoided in the treatment of benign rectal lesions. The technique of TEM was first developed and applied to lesions in the mid and upper rectum which were either difficult to reach, or completely inaccessible via traditional transanal approaches. Several authors postulate that TEM is both more effective and will lead to better long term outcomes when used to treat benign rectal lesions. The reason for this is the clear advantage of superior visibility, particularly of the proximal margin. Additionally the rate of piecemeal excision is greatly reduced with this approach [8].
Moore et al. reviewed their data regarding 171 patients who underwent TEM versus traditional transanal excision and they report a statistically significant decrease in the rate of local recurrence following TEM in the resection of benign rectal adenomas [8]. The authors attribute this decrease in local recurrence to the removal of nonfragmented TEM specimens, as well as the decreased positive margin rate associated with TEM. This data confirms the fact that TEM affords the surgeon a better view of the operative field, as well as better instrumentation promoting both complete excision and adequate resection margins necessary to prevent recurrence.
Another institutional review of 15 years experience in TEM resection of rectal adenomas by Guerrieri et al. revealed in addition to decreased recurrence rate, TEM is also a low morbidity procedure with no reported mortality. TEM provides for the safe removal of rectal adenomas up to 20 cm from the anal verge. The observed rate of recurrence in this study was 4 % at 84 months follow-up [9].
Malignancy
The indications for local excision with or without TEM in the treatment of early stage rectal cancer have been debated for years in the literature. A large number of trials have reported high local recurrence rates for rectal cancer treated with local excision. In the 1990s, Bleday and Steele reported on 48 patients treated with full-thickness local excision, resulting in 10 % failure rate for T1 cancers and a 40 % failure rate for T2 cancers [10]. Later that decade, the CALBG group, in a study of 110 patients treated with local excision, reported a similar failure rate for T1 cancers of 6 % and a 14 % failure rate for T2 cancers. Notably, 15 % of resections had positive margins [11]. Rothenberger’s group out of the University of Minnesota reported in 2000 that of 108 patients treated with local excision, 18 % of T1 cancers recurred as well as 47 % of T2 cancers [12]. In 2005, the same group further reported on 151 patients treated with local excision with failure rates of 19 % for T1 and 45 % for T2 cancers [13]. Also in 2005, the Cleveland Clinic published a study of 52 local excisions and a failure rate of 29.4 % for T1 lesions [14]. The data from these trials led Rothenberger et al. to conclude that local excision alone is inappropriate treatment for T2 rectal cancer [12].
The Norwegian rectal cancer group published a study in 2005 examining T1 cancers treated with radical resection versus local excision. Of the 256 patients treated with radical surgery, 6 % had local recurrence. Node positivity occurred in 11 % of T1 cancers, and as expected with early cancers, 100 % of the resections were R0. On the other hand, 38 patients were treated with local excision. The failure rate for these patients was 12 % and there was a 17 % R2 resection rate [15]. Interestingly, the local excision failure rate in the experiences cited above is similar to the node positivity rate for T1 cancers treated with radical resection. Based on the high rate of positive margins in this group, it is surprising that the local recurrence rate is not even higher. Lymph node involvement for T1 cancers after radical resection is 6–12 % and failure rate after local excision in these trials is 6–29 % in the reports just mentioned. There are two possible explanations for this: persistence of untreated cancer in lymph nodes not resected during local excision or implantation of tumor cells at the time of surgery due to incomplete resection or handling of the tissue transanally. TEM surgery coupled with neoadjuvant therapy addresses both of these issues.
A pressing question when considering treatment options for rectal cancer remains: is there a role for radiation in stage I rectal cancer? Should preoperative radiation be utilized in early stage cancers of the rectum in conjunction with local excision? The MRC CR07 trial, a multicenter randomized study, compared preoperative radiation therapy to selective use of postoperative radiation therapy in 1,350 rectal cancer patients treated with radical resection. They found improved local recurrence rates, all of which were statistically significant, for cancers treated with radiation preoperatively—T1: 1.9 %, T2: 1.9 %, T3: 7.4 %; for cancers treated selectively with postoperative radiation—T1: 2.8 %, T2: 6.4 %, T3: 15.4 %. The trial concluded that there is a significant improvement in local control for stages I, II, and III rectal cancer after preoperative radiation therapy, and they advocate the use of preoperative treatment for all stages [16, 17].
Our experience represents the first in the world performing local excision in conjunction with preoperative radiation therapy in 1984 at Jefferson University Hospital [18]. We have continued to employ the Marks and Mohiuddin method and in 2004, reported on an experience with 44 patients with T2N0 disease in the distal 7 cm of the rectum, all treated with local excision. Twenty five percent of these patients were treated with TEM; the remainder were treated using transanal approaches. We found an overall local recurrence rate of 6.9 % and a 5-year survival of 91 %. There was a complete response after neoadjuvant therapy in 23 % of patients, none of which had a local recurrence [19]. We further compared our experience with 73 T2 rectal cancers treated neoadjuvantly followed by TEM versus total mesorectal excision (TME). The local recurrence rate in the TEM group was 3.3 % compared to 2.3 % in the TME group. This difference was not statistically significant. Similarly, the difference in 5-year survival did not reach statistical significance: 95 % in the TEM group versus 97 % in the TME group [20]. Lezoche, as mentioned elsewhere, has published comparable results for cancers treated neoadjuvantly followed by full-thickness local excision. His local recurrence rate was 5 % and he saw an 89 % survival rate [21].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








