Dennis M. Jensen1,2,3,4 1 David Geffen School of Medicine, UCLA, Los Angeles, CA, USA 2 Department of Medicine – Gastroenterology Division, West Los Angeles Veterans Administration and Ronald Reagan UCLA Medical Center, Los Angeles, CA, USA 3 CURE: Digestive Diseases Research Center‐Human Studies CORE, Los Angeles, CA, USA 4 VA/CURE GI Hemostasis Research Unit, Los Angeles, CA, USA Acute gastrointestinal (GI) bleeding requiring emergency endoscopic intervention is a common high‐risk medical and surgical problem. Traditional apprentice‐type training for such GI events is often inadequate because of their unpredictable frequency, usual off‐hours occurrence, urgency to complete, and potential risks of poor outcomes or complications. Similar problems occur in other medical specialties such as cardiology or trauma surgery. The development of the Advanced Cardiac Life Support (ACLS) and Advanced Trauma Life Support (ATLS) programs has facilitated the training of health‐care professionals to better prepare for those infrequent events. Both programs allow trainees to rehearse resuscitation scenarios outside of the emergency clinical environment until proper management is learned. A similar program would be useful for training about the management of acute GI bleeding, but national or international programs to do this are needed but lacking. When learning about the endoscopic management of GI bleeding during an emergency case, the number of different potential diseases and their acute management seems daunting. However, if the diagnoses are categorized by GI organ location, anatomy, lesion vascularity, and comorbidities and the hemodynamic condition of the patient are considered, similarities in patient medical treatment and endoscopic hemostasis become more manageable (Table 16.1). For endoscopic management of GI bleeding, the trainee should focus on a differential diagnosis of the potential lesions, the type of vascular supply, and what type of endoscopic management strategy to use for each type of lesion. For endoscopic treatment of GI bleeding, there are currently four major categories—topical, injection, thermal, and mechanical. This chapter focuses on defining the processes and techniques of learning the skills required to successfully achieve endoscopic GI hemostasis. It does not explore indications and contraindications for the procedures or efficacy since that material is covered in a general textbook of gastroenterology. Instead, the chapter describes how one trains in endoscopic hemostatic techniques by outlining both the prerequisite and necessary knowledge and technical skills required to become proficient. The appropriate settings to learn hemostatic strategies are also discussed as are the benefits and limitations of simulators, current hemostasis guidelines, and use of additional learning tools and aids. Finally, assessment of competency in endoscopic hemostasis and maintenance of skills is reviewed. Hemostasis as defined in this chapter is the cessation of active bleeding and prevention of rebleeding with an endoscopic intervention. It has an added dimension of going beyond visual endoscopic hemostasis to now include monitoring and obliterating underlying blood flow. With success in all these goals, clinical outcomes patients have been shown to improve. However, failure to achieve all of these goals often results in rebleeding and need for further intervention. Although they are critically important, neither the medical management nor the clinical outcomes of GI bleeding are the primary focus of this chapter. The first step to becoming proficient in endoscopic hemostasis is to acquire a fundamental knowledge of the pathophysiology and clinical presentation of different GI bleeding lesions. This cognitive learning is gained through studying textbooks of gastroenterology, reviewing literature about GI hemorrhage, and participating in postgraduate training focused on diseases of the GI tract. Such knowledge is critical so that the endoscopist who is trying to learn GI hemostasis management at the bedside can discuss a focused differential diagnosis and the expected lesion vascular anatomy that will guide the therapeutic and endoscopic plans. The following examples illustrate the importance of accurate differential diagnoses and understanding lesion blood supply. Example 1: A 55‐year‐old cirrhotic patient presents with hematemesis and melena. A knowledgeable endoscopist should suspect this patient to have a lesion related to portal hypertension and venous blood flow such as esophageal or gastric varices or a Mallory–Weiss tear [1]. The trainee should know that bleeding from these lesions is venous and probably related to portal hypertension. The most likely treatments that should be anticipated and prepared for are band ligation, sclerotherapy, and injection of glue (cyanoacrylate) (Table 16.2). These endoscopic treatments are in addition to concomitant hemodynamic resuscitation, octreotide drip, treatment of coagulopathies, antibiotics, and management of comorbid conditions (1). Based upon the potential diagnoses, the GI team should be instructed to equip the emergency endoscopy cart with the proper supplies and be prepared to use them. Other important pre‐endoscopic decisions are about airway protection (in patients with altered mental or respiratory status and to prevent aspiration of blood) and methods to remove blood or clots and to improve visualization prior to and during the endoscopy. The timing of the procedure and also the type of anesthesia are important. It may be advisable to perform the procedure in the ICU either with tracheal intubation or with an anesthesiologist with MAC anesthesia. Other decisions to make prior to the emergency endoscopy are what types of endoscopes (therapeutic and diagnostic), irrigators, and accessory devices to bring to the bedside. Finally, once the procedure is underway, the endoscopist must communicate closely with the GI team to ensure proper monitoring of the patient, adequate sedation, and accurate application of hemostatic devices. Table 16.1 Sources of GI hemorrhage categorized by location and whether the patient has cirrhosis* or not based on medical history. * Applicable in patients with cirrhosis. Example 2: A 65‐year‐old patient with multiple comorbidities (without cirrhosis) presents with painless melena, hypotension, and severe anemia while on chronic aspirin and clopidogrel for secondary prevention of cardiovascular events. With this history, the most likely diagnosis is an aspirin‐induced ulcer with or without stigmata of recent hemorrhage (SRH). Furthermore the experienced clinician and endoscopist know that the method of hemostasis recommended as the standard of care and the risk stratification for rebleeding prior to endoscopic hemostasis depend upon major vs. minor SRH. For ulcers, the usual findings related to SRH (with their prevalence and risk of continued bleeding or rebleeding on medical management alone without endoscopic treatment) are major SRH [spurting arterial bleeding (5%/80–90%), a nonbleeding visible vessel (NBVV) (20%/40–50%), adherent clot (10%/25–35%)], lesser SRH [oozing bleeding without other SRH (10%/20–30%) or a flat spot (5%/20%)], and vs. no SRH [clean ulcer base (50%/<5%)] [2]. It is much less likely that this patient with severe acute bleeding and hemodynamic instability has an upper GI (UGI) neoplasm, varices, an angioma syndrome, or a diffuse lesion such as esophagitis. The endoscopist and GI team must be prepared to perform emergency endoscopy with a therapeutic panendoscope (with separate channel for target irrigation and a large suction port to remove blood and clots prior to focal endoscopic treatment). They should have concluded before the endoscopy that the most likely source of bleeding is arterial from an ulcer or Dieulafoy’s lesion. They should expect to use combination therapy with injection of dilute epinephrine (1:20,000 dilution in saline) followed by thermal coagulation, standard hemoclipping, or large over‐the‐scope clip (OTSC) as the most effective methods of definitive hemostasis (Table 16.2) [2–4]. With current endoscopic techniques using SRH and visually guided outcomes, the rebleeding rates are still 15–25% for major SRH. The rebleeding rates can be significantly decreased by Doppler endoscopic probe (DEP) monitoring of arterial blood flow and using this as a guide to definitive hemostasis [5]. (See later section on DEP.) Example 3: A 70‐year‐old patient presents with severe hematochezia, hypotension, and anemia. An experienced endoscopist knows that an urgent colonoscopy after purge is recommended as the best way to make the diagnosis and apply successful hemostasis to focal lesions such as a diverticula bleed, or post‐polypectomy induced ulcers, a rectal ulcer, or angiomas [5, 6]. An UGI source should be considered for patients with cirrhosis, a history of hematemesis or melena, and a positive nasogastric aspirate and for patients who develop severe hematochezia as inpatients [6, 7]. In contrast, for the elderly patient who develop hematochezia at home as an outpatient, the most likely location of the bleeding lesion is the colon. In such a patient, the differential diagnosis of lesions includes, in descending order of prevalence, a diverticular bleed, ischemia, internal hemorrhoids, rectal ulcers, colitis, delayed post‐polypectomy ulcer (if recent polypectomy), or an angioma syndrome [6–10]. After thorough cleansing of the colon with purge to remove all clots, blood, and stool, urgent colonoscopy and treatment of focal lesions are recommended. Lesions that are amenable to colonoscopic hemostasis include those with underlying arteries and SRH (definitive diverticular bleeds and ulcers of any type), angioma syndromes (radiation or angiodysplasia), or venous‐type bleeding (internal hemorrhoids). The three general types of endoscopic accessories that will be required for treatment of this spectrum of colon lesions are thermal probes, hemoclips with or without epinephrine injection, and banding (for bleeding internal hemorrhoids). Table 16.2 CURE Hemostasis Group basic guide to anatomy, type of blood supply, and location of hemostasis. SRH, stigmata of recent hemorrhage; NBVV, nonbleeding visible vessel; EMR, endoscopic mucosal resection; RBL, rubber band ligation; HP, heater probe; Combo, combination injection of epinephrine and either MPEC/HP or hemoclipping; PPIU, post‐polypectomy induced ulcer; AVM, arteriovenous malformation; GEJ, gastroesophageal junction; PHTN, portal hypertension; OWR, Osler–Weber–Rendu syndrome; WMS, watermelon stomach or gastric vascular ectasia (GAVE); XRT, radiation telangiectasia. These examples illustrate the in‐depth knowledge that is required to formulate an accurate differential diagnosis for patients with GI bleeding; to plan for the timing, protection of the airway, and type of anesthesia for a safe emergency procedure; and to anticipate and bring to the bedside the necessary equipment and accessories for the procedure. Acquiring this knowledge is a prerequisite before the trainee attempts to learn about the technical aspects of endoscopic management of GI bleeding [1–10]. Emergency endoscopic hemostasis is an advanced procedure and should not be attempted by a novice, untrained endoscopist. Once the cognitive aspects about sources of bleeding as discussed above are mastered along with the knowledge of medical management and resuscitation, substantial technical knowledge and skill related to the procedure are necessary to be able to successfully manage patients with severe GI hemorrhage. Requisite knowledge includes understanding the proper setup and function of the endoscopic equipment, such as a standard gastroscope, therapeutic gastroscope (single and double channel), duodenoscope, enteroscopes (standard push and balloon enteroscopes), and colonoscope. Also needed are a thorough knowledge of anesthesia for emergency procedures (including IV moderate sedation or MAC anesthesia) and protection of the airway to prevent aspiration. Finally, a basic understanding of the principles of electrosurgery is necessary, although a more thorough review is critical to master successful and safe hemostatic techniques. Prerequisite technical skills include the safe administration of moderate sedation, ability to minimize the risk of aspiration, and application of airway rescue management strategies if necessary (such as the use of oral airway adjuncts, bag ventilation, and endotracheal intubation). Mastery of basic endoscopic manipulation is also required. This can be better understood if the skill is deconstructed into component steps. It is recommended that any practitioner who wants to learn endoscopic hemostasis skills should first have the cognitive and technical knowledge discussed above prior to starting their hemostasis training, including performing bedside cases. In contrast to the breadth of knowledge required to understand the pathophysiology and current medical–surgical management of all causes of GI bleeding, the knowledge and skills required to actually perform endoscopic hemostatic maneuvers are less expansive. GI hemostasis can more easily be broken down into components with common strategies. Each of these strategies requires a specific fund of knowledge and technical skill to be successfully applied so that good clinical outcomes result. No matter what hemostatic strategy is applied or what source of GI bleeding is treated, there is a common knowledge base required to successfully perform endoscopic hemostasis. This certainly begins with a thorough understanding of the most common GI lesions, their anatomy, and their vascular supply (Table 16.2) but also includes a familiarity with the endoscopes and endoscopic accessories and devices [1, 11]. The endoscopist or trainee should not expect to rely on an endoscopic assistant for an understanding of the proper use, deployment, or technical differences of various devices for endoscopic hemostasis. Skilled endoscopists must understand the proper use of all endoscopes and accessories that may be needed for control of bleeding in emergency or elective cases of GI hemorrhage. They should be able to teach trainees and members of endoscopy team about all the technical settings, utilization, advantages, and disadvantages of all the equipment and accessories. Both experts and trainees should also examine new technologies and treatments as these are reported upon and continuously read the literature to keep up to date on the efficacy, safety, impact on clinical outcomes, and guidelines for deployment of new devices and management strategies. In addition, trainees must develop the pattern recognition skills to correctly identify the different types of lesions that bleed, their GI location, and the SRH. They should understand both the prognostic significance and the appropriate management decisions for each type of lesion and different SRH (Figure 16.1). Common skills sets for endoscopic hemostasis include those described in the prerequisites above. In addition, an ability to use different strategies to clear the endoscopic field of blood is essential. These include use of powerful irrigation systems, endoscopes with large bore accessory channels, caps or hoods, and different suction adjunct (such as BioVac). A standard upper endoscope typically has a 2.8‐mm accessory channel, whereas a therapeutic upper endoscope will have a 3.7‐ or 3.8‐mm accessory channel depending on the manufacturer. An adult colonoscope typically has a 3.7–3.8‐mm accessory channel but can increase to 4.2 mm. Modern therapeutic duodenoscopes have a 4.2‐mm accessory channel. Most current upper endoscopes and colonoscopes also have a separate port for water jet irrigation operated by a pump and separate foot pedal. After injection of dilute epinephrine, using a snare to remove an adherent clot is another skill required for gaining endoscopic hemostasis. Also, changing patient position can aid in the examination of different areas of the GI tract. For example, when performing upper endoscopy in the standard left lateral position, gastric fluid, clots, and blood pool in the fundus and can obscure bleeding lesions in the upper stomach or cardia. By rolling the patient into right lateral position, the fluid and clots will usually move toward the antrum, leaving a clearer view of the fundus and cardia (Figure 16.2). As a cautionary note, elevation of the thorax to 45° or more and protection of the airway with an overtube or intubation before rotating the patient onto the right side are highly recommended to prevent aspiration. This simple strategy can be very effective in identifying a bleeding source in the stomach during emergency endoscopy, such as Dieulafoy’s lesions or gastric varices (GV). The ability to effectively communicate with the rest of the endoscopy team is also critical for successful endoscopic management of GI bleeding, particularly during emergency procedures. Proper use of the endoscopic accessories requires coordination with the assistant, while the nurse protects the airway and carefully monitors the patient’s vital signs, oxygenation, and sedation. A rehearsal of how to utilize the equipment and accessories that are unfamiliar to the team is critical before starting the procedure. A review of the management plan and each member’s duties during the endoscopy is also extremely helpful prior to introducing the endoscope. Pre‐procedure planning should also ensure that sufficient supplies are available on the emergency cart and that the team is prepared for the use of more than one hemostasis modality in the event that combination therapy is needed. Injection of a drug (e.g., vasoactive or sclerosant) with a needle is often useful to slow arterial bleeding or control spurting variceal bleeding (Figure 16.3). Understanding what substance to inject for different lesions, where to inject it (e.g., intravariceal or paravariceal for varices or around the SRH in the ulcer base), and the complications of injection are important. One example is epinephrine—the most common substance injected for nonvariceal bleeding. It is typically used at 1:10,000 or 1:20,000 concentrations but may be diluted more with saline if lower concentrations are desired. It is injected as 1–2‐cc aliquots into the submucosal layer, directly into the base of the ulcer or lesion around the SRH, typically in four quadrants. Common side effects are transient tachycardia and hypertension. Table 16.3 lists substances used for injection into bleeding lesions [12]. There is a major limitation of injection for bleeding ulcers. As monotherapy, injection therapies for ulcers with major SRH have been reported to have significantly higher rebleeding rates than thermal coagulation, hemoclips, or combination therapy with epinephrine and coagulation or hemoclipping [13]. Figure 16.1 Stigmata of recent hemorrhage. (a) “Nipple sign” or platelet plug on esophageal varix. (b) Ulcer with flat black spot. (c) Ulcer with adherent clot. (d) Ulcer with oozing‐type bleeding. (e) Ulcer with pearly clear visible vessel in the 2:00 position with subsequent spurting observed 3 days later after failure to recognize and coagulate this stigmata of recent hemorrhage (SRH). Trainees must learn to recognize, correctly classify, and understand how to use SRH for risk stratification, selection of hemostasis technique, and application of the endoscopic treatment for different types of GI bleeding lesions. Figure 16.2 Effect of changing patient position on gastric liquid. In the left figure is the stomach with fluid for the patient in the left‐side down position. The fundus and upper stomach are obscured by blood or fluid. Rotation of the patient to the right‐side down position and elevation of the thorax to 30–45° after protection of the airway by intubation or an endoscopic overtube usually moves blood, fluid, and clots into the antrum. This allows the endoscopist to visualize and successfully treat bleeding lesions such as gastric varices, ulcers, or Dieulafoy’s lesions in the fundus and upper stomach [1, 2]. Figure 16.3 Single‐use sclerotherapy needles. Two different injector needles—the top one for nonvariceal hemostasis and the bottom needle for variceal sclerotherapy. Table 16.3 Injectables for endoscopic hemostasis. Applying thermal energy to a bleeding lesion either alone or in combination with epinephrine injection therapy is also commonly used for endoscopic hemostasis [1, 2]. A thorough knowledge of the principles of electrosurgery is critical for this technique. A comprehensive review of this topic is beyond the scope of this chapter but is included in a separate chapter in this book (see Chapter 12). Some basic principles of electrosurgery and types of generators will be reviewed however. Cauterizing a lesion with electromechanical energy (electrocautery) can be achieved by applying an alternating electrical current to the target tissue via an accessory such as a snare, probe, or forceps. Patients are not “shocked” by this energy the way they would be by coming into contact with a common wall outlet because it is delivered at a very high frequency in the radio wave spectrum (radiofrequency) and a grounding pad is used with monopolar coagulation. All electrocautery requires a complete circuit to be delivered to the target tissue. There are two ways to complete this circuit: monopolar or bipolar electrocoagulation. Monopolar electrocautery completes the circuit by the electrical current passing through the patient to a grounding pad or plate and back to the generator (Figure 16.4). The current in bipolar or multipolar electrocoagulation (MPEC) passes through the affected tissue only at the tip of the probe or accessory and does not require a grounding pad (Figure 16.5). Monopolar electrocautery can penetrate deeply into tissues and is effective for hemostasis, but the risks are deep thermal injury, transmural injury, and possible perforation in thin‐walled gut structures. Bipolar energy does not penetrate as deeply and can be less effective in endoscopic hemostasis, unless firm tamponade pressure is applied to achieve coaptive coagulation [1, 2]. A thorough knowledge of these principles along with proper energy settings on the generator used for electrocautery is critical for the safe application of this technology (see Tables 16.6–16.8). Argon plasma coagulation (APC) is a special version of monopolar electrocautery that requires additional understanding. Argon gas, when “electrified,” converts to plasma that will conduct monopolar current. APC is useful when superficial tissue coagulation can be applied to a large surface area without touching the tissue. This prevents buildup of debris on the probe tip and allows for the expeditious “painting” of large areas, such as in watermelon stomach, radiation telangiectasia, or angiodysplasia (Figure 16.6). Understanding the unique energy settings and argon gas flow rates for operating an APC probe is essential. Being mindful to constantly aspirate the argon gas from the GI tract is also important. Limitations of APC for GI hemostasis must also be understood. For example, touching the electrode tip directly to the tissue results in monopolar coagulation, potentially transmural coagulation, and higher risk of perforation. In addition, coaptive coagulation (applying pressure with the electrocautery probe to collapse the walls of the bleeding vessel together for sealing) is not possible with APC, which limits its hemostasis application to superficial coagulation of AVMs or oozing bleeding from very small vessels [1, 2]. Figure 16.4 Monopolar electrocautery. Current passes from the tip of the electrode through the patient to the grounding pad and back to the grounded generator. A grounding pad is required and deep tissue injury may occur. This is also the type of radiofrequency monopolar generator and current for argon plasma coagulation (APC). (Reproduced with permission from ERBE USA Inc.) Figure 16.5 Bipolar or multipolar electrocautery. Current passes around the tip of the probe or (forceps) from one plate (or jaw) to the other and back to the generator. A grounding pad is not required, and superficial coagulation usually results but is deeper when firm coaptive pressure is applied. (Reproduced with permission from ERBE USA Inc.) Another method of applying thermal energy to tissue in the GI tract is with a heater probe. This device heats up rapidly and transfers a preselected amount of heat across a Teflon‐tipped probe to the tissue. The heater probe does not apply electrocautery to the tissue, but cauterizes it by generating heat at its tip. Deep tissue injury is possible if repeated pulses are applied. Understanding the energy settings for different types of lesions, the size of probe to use, methods of irrigation, and conformity of the tip are all important for safe and successful application of heater probe for bleeding GI lesions (Table 16.6). Heater probe has been taken off the market in the United States but may still be available in some other countries. Figure 16.6 Figure 16.7 (a) Coaptive coagulation. Firm pressure (tamponade) to stop active bleeding and interrupt the blood flow of the underlying artery is first applied. Then coagulation (MPEC) or heat (heater probe) for long pulse duration. Utilizing the large thermal probes (heater or MPEC probes) will coagulate tissue and can weld the walls of underlying arteries as large as 2 mm together. (Reproduced from Jensen DM, Machicado GA: Endoscopic hemostasis of ulcer hemorrhage with injection, thermal, or combination methods. Tech Gastrointest Endosc 2005;7:124131, with permission from Elsevier.) (b) Visible vessel pre‐coaptive and (c) post‐coaptive coagulation with bipolar probe. The required knowledge for proper endoscopic band placement on esophageal varices is similar with a distal to proximal application and knowledge of stigmata of recent variceal hemorrhage that will aid in directing where to apply the bands most effectively. For large over‐the‐scope hemoclips, the loading of the endoscope is similar to banding, but more training and skill are required to successfully utilize these for emergency hemostasis of bleeding ulcers or Dieulafoy’s lesions in the foregut. The OTSC grasps more tissue, can be deployed on firm ulcers with scarring, and has been reported to obliterate underlying arterial blood flow (when deployed with the SRH in the center of the large hemoclip) than standard hemostasis [16]. Consequently when OTSC is successfully deployed on ulcers or Dieulafoy’s lesions, the rebleeding rates are significantly lower than standard hemoclips or MPEC [17, 18].
16
Training in GI Hemostasis
Introduction
Prerequisite cognitive knowledge required prior to learning GI endoscopic hemostatic techniques
Esophagus
Varices*
Mallory–Weiss tear*
Esophagitis
Ulcer
Mass
Postsurgical anastomosis
Post‐banding ulcer (of varices)*
Stomach
Varices*
Ulcer
Dieulafoy
Neoplasia—polyps or cancer
Angioma syndromes*
Postsurgical anastomosis
Post‐endoscopic intervention (EMR or banding)
Duodenum
Ulcer
Angiomas*
Mass or neoplasm
Postsurgical anastomosis
Post‐endoscopic intervention (EMR or sphincterotomy)
Small bowel
Ulcer
Angiomas*
Mass or neoplasm
Postsurgical anastomosis or ulcer
Colon
Diverticulum
Mass or neoplasm
Angiomas*
Internal hemorrhoids
Post‐endoscopic intervention
(delayed of post‐polypectomy ulcer bleed)
Postsurgical anastomosis
Types of GI lesions
Type of blood supply
Usual locations
Usual stigmata of recent hemorrhage (SRH)
Treatment
Ulcers (peptic, ischemic, infections, postsurgical, post‐EMR)
Arterial
All GI
Any SRH
Combo
Dieulafoy’s lesion
Arterial
Gastric fundus, UGI
Spurting, NBVV
Combo
Mallory–Weiss tears
Arterial (unless with portal hypertension)
GEJ
Ooze, spurt, clot, or clean
HC if no PHTN, RBL/sclero if PHTN
Ulcerated polyps or PPIUs
Arterial
All GI
Any SRH
Resect polyp, Combo—PPIU
Varices
Venous—PHTN
UGI (colon, anastomoses)
Spurting, clot, NBVV, or none
RBL or sclero Rx
Internal hemorrhoids
Venous
Rectum
Ooze or none
RBL
Angioma syndromes (OWR, WMS, XRT, angiodysplasia, and idiopathic angiomas)
AVM
All GI
Oozing, clot, spot, or clean lesions
Thermal—MPEC, HP, or APC
Cancers—ulcerated
Neovascularity
All GI
Oozing
Possible hemoclipping and tattooing, surgery
Inflammatory lesions (esophagitis, celiac disease, or colitis)
Capillaries
All GI
Oozing
Medical
Infections
Capillaries
All GI
Oozing
Medical
Prerequisite technical knowledge and skills required to learn endoscopic hemostasis
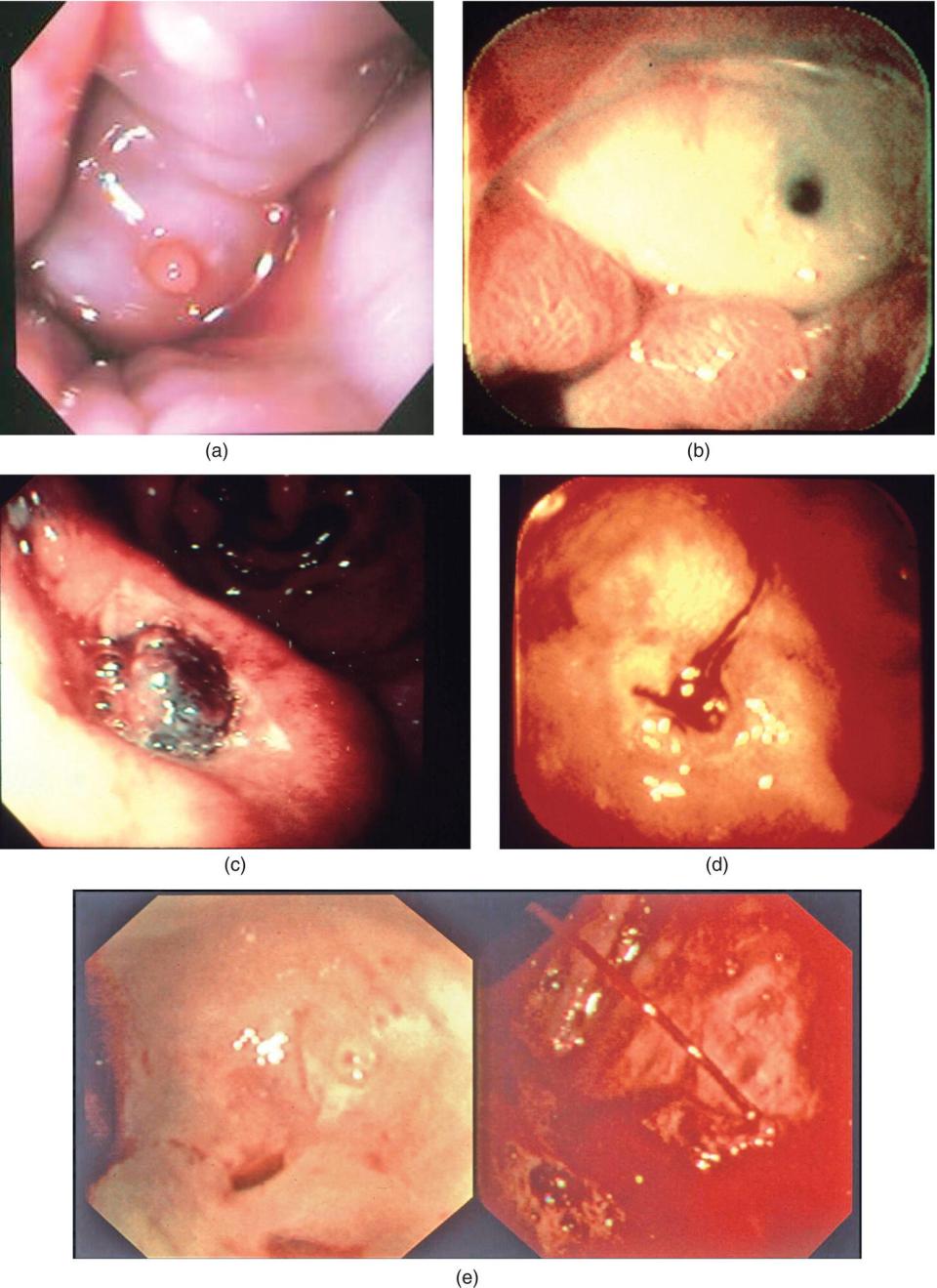
 Both upper and lower endoscopy require a common skill set that includes the ability to provide scope traversal, four‐way tip deflection, and torque to deliver the endoscope to the desired location. Endoscopy also requires the correct use of insufflation and target irrigation to properly identify lesions and access various areas of the GI tract. Targeting is another critical skill that enables skilled endoscopists to deliver the endoscopic accessory onto the lesion of interest with proper orientations (see Video 16.1). Upper endoscopy requires the additional skill of being able to traverse the upper sphincter and pylorus. Finally, an endoscopist must be able to communicate well with the rest of the endoscopic team in order to make accurate diagnoses and to perform successful endoscopic therapies.
Both upper and lower endoscopy require a common skill set that includes the ability to provide scope traversal, four‐way tip deflection, and torque to deliver the endoscope to the desired location. Endoscopy also requires the correct use of insufflation and target irrigation to properly identify lesions and access various areas of the GI tract. Targeting is another critical skill that enables skilled endoscopists to deliver the endoscopic accessory onto the lesion of interest with proper orientations (see Video 16.1). Upper endoscopy requires the additional skill of being able to traverse the upper sphincter and pylorus. Finally, an endoscopist must be able to communicate well with the rest of the endoscopic team in order to make accurate diagnoses and to perform successful endoscopic therapies.
Required technical knowledge and skills to be proficient in endoscopic hemostasis
Common knowledge and skills for all hemostatic strategies
Knowledge
Skill
Specific hemostatic strategies
Injection
Knowledge


Skill
 One of the reasons injection therapy is so common is that it is technically easy to perform with little chance for an adverse event, at least with dilute epinephrine or other vasoactive injectants. The skills required to perform sclerotherapy include an ability to pass the device down the working channel, deliver it to the target, extrude the needle, and insert the needle into the lesion or near the SRH and into the desired tissue plane. Ongoing communication with the assistant during emergency endoscopy for deployment of the needle and injection of the sclerosant or epinephrine is critical (see Video 16.2).
One of the reasons injection therapy is so common is that it is technically easy to perform with little chance for an adverse event, at least with dilute epinephrine or other vasoactive injectants. The skills required to perform sclerotherapy include an ability to pass the device down the working channel, deliver it to the target, extrude the needle, and insert the needle into the lesion or near the SRH and into the desired tissue plane. Ongoing communication with the assistant during emergency endoscopy for deployment of the needle and injection of the sclerosant or epinephrine is critical (see Video 16.2).
Nonvariceal lesions with SRH
Varices
Epinephrine (1:10,000 or more dilute)
Yes
No
Ethanol 99.5%
Yes
Yes
Hypertonic (50%) dextrose
Yes
No
Polidochanol 0.5–3%
Yes
Yes
Ethanolamine oleate 5%
No
Yes
Sodium morrhuate 5%
No
Yes
Sodium tetradecyl sulfate 1 or 3%
No
Yes
Thermal
Knowledge


Skill
 Application of a heater probe, bipolar probe, or another contact electrocautery device by touching the probe tip to the tissue is called contact coagulation. When using these devices in the UGI tract for hemostasis, it is best to apply the principle of coaptive coagulation (Figure 16.7) (see Video 16.3). This technique requires applying the thermal probe directly on the actively bleeding site or SRH with enough pressure to stop the bleeding by interrupting blood flow in the underlying vessel and collapsing its walls against each other [2]. When the heat or electrocautery is applied, the vessel walls can be sealed together, and definitive hemostasis is achieved. Having the skill to deliver the thermal device to the target with the proper angle and force is a necessary skill for effective thermal hemostasis and coaptive coagulation [1, 2]. To do this requires the ability to keep the endoscope in a stable position with the probe tip close to the endoscope tip to maximize the ability to apply firm and steady pressure. Laboratory studies have reported that arteries as large as 2 mm in diameter can be sealed with heater probe or bipolar (or multipolar) probes using coaptive coagulation [14]. However, recent clinical studies of ulcers have reported that residual arterial blood flow is detectable in about 25% of patients with major SRH treated with visually guided coaptive coagulation at endoscopy [5, 15]. These patients have a high risk of rebleeding.
Application of a heater probe, bipolar probe, or another contact electrocautery device by touching the probe tip to the tissue is called contact coagulation. When using these devices in the UGI tract for hemostasis, it is best to apply the principle of coaptive coagulation (Figure 16.7) (see Video 16.3). This technique requires applying the thermal probe directly on the actively bleeding site or SRH with enough pressure to stop the bleeding by interrupting blood flow in the underlying vessel and collapsing its walls against each other [2]. When the heat or electrocautery is applied, the vessel walls can be sealed together, and definitive hemostasis is achieved. Having the skill to deliver the thermal device to the target with the proper angle and force is a necessary skill for effective thermal hemostasis and coaptive coagulation [1, 2]. To do this requires the ability to keep the endoscope in a stable position with the probe tip close to the endoscope tip to maximize the ability to apply firm and steady pressure. Laboratory studies have reported that arteries as large as 2 mm in diameter can be sealed with heater probe or bipolar (or multipolar) probes using coaptive coagulation [14]. However, recent clinical studies of ulcers have reported that residual arterial blood flow is detectable in about 25% of patients with major SRH treated with visually guided coaptive coagulation at endoscopy [5, 15]. These patients have a high risk of rebleeding.
 (a) Argon plasma coagulation (APC). Non‐touch technique with APC. Refer also to Figure 16.4. A grounding pad is required, and if tissue is touched with the tip of the catheter, monopolar coagulation (which may be deep) occurs. (b) Gastric antral vascular ectasia (GAVE) pre‐ and post‐APC treatment painting all visible stripes with noncontact thermal coagulation (see Videos 16.6 and 16.7).
(a) Argon plasma coagulation (APC). Non‐touch technique with APC. Refer also to Figure 16.4. A grounding pad is required, and if tissue is touched with the tip of the catheter, monopolar coagulation (which may be deep) occurs. (b) Gastric antral vascular ectasia (GAVE) pre‐ and post‐APC treatment painting all visible stripes with noncontact thermal coagulation (see Videos 16.6 and 16.7).
Mechanical
Knowledge
 The third general method of gaining endoscopic hemostasis is mechanical techniques including endoscopic through the scope hemoclips, large over‐the‐scope hemoclip, band ligation devices, or ligating loops. There are different manufacturers for these devices, so it is essential to become familiar with the design and function of each before using these in patients (see Video 16.4). In addition, the knowledge required to place endoscopic clips includes the principle of working distal to proximal on a lesion so as not to cross clips that have already been applied and risk dislodging them and a desire to apply the clip as perpendicular to the target as possible. A successful strategy in applying hemoclips to nonvariceal lesions with SRH and underlying arteries (see Table 16.2) is to place one hemoclip well into the submucosa at 45–75° on each side of the SRH [2]. Alternately, using DEP to detect the location of the artery in the ulcer base and placing hemoclips on the artery on each side of the SRH to obliterate underlying blood flow will significantly reduce the risk of rebleeding and improve other clinical outcomes [1, 3, 5, 10, 15]. Most single‐use hemoclips are rotatable, which helps orient the clip on the target prior to deployment.
The third general method of gaining endoscopic hemostasis is mechanical techniques including endoscopic through the scope hemoclips, large over‐the‐scope hemoclip, band ligation devices, or ligating loops. There are different manufacturers for these devices, so it is essential to become familiar with the design and function of each before using these in patients (see Video 16.4). In addition, the knowledge required to place endoscopic clips includes the principle of working distal to proximal on a lesion so as not to cross clips that have already been applied and risk dislodging them and a desire to apply the clip as perpendicular to the target as possible. A successful strategy in applying hemoclips to nonvariceal lesions with SRH and underlying arteries (see Table 16.2) is to place one hemoclip well into the submucosa at 45–75° on each side of the SRH [2]. Alternately, using DEP to detect the location of the artery in the ulcer base and placing hemoclips on the artery on each side of the SRH to obliterate underlying blood flow will significantly reduce the risk of rebleeding and improve other clinical outcomes [1, 3, 5, 10, 15]. Most single‐use hemoclips are rotatable, which helps orient the clip on the target prior to deployment.
Skill
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






