Traditional Chinese medicines are sometimes used as an adjunct to radiotherapy or chemotherapy for esophageal cancer. These medicines may have a benefit on the survival and quality of life of patients who have advanced esophageal cancer. Evidence from current studies appears weak due to methodological limitations. Due to conflicting reports, it is difficult to argue for or against the use of traditional Chinese medicines as a treatment for esophageal cancer.
Esophageal cancer is the seventh leading cause of cancer death worldwide. It has two subtypes—squamous carcinoma and adenocarcinoma. The occurrence of esophageal cancer varies by geographic area, ethnic group, and gender. The incidence of esophageal cancer in particular areas of northern Iran is as high as 30 to 800 cases per 100,000 persons and in some areas of southern Russia, northern China, and the United States approximately three to six cases per 100,000 persons. It is generally more common in men than in women; the incidence in males is seven times higher than in females.
Esophageal cancer is highly treatable by surgery in its earliest stages but is usually fatal in the advanced stages. The application of treatment is dependent on the stage of the disease at diagnosis. Esophageal resection (esophagectomy) is used in patients who are suitable for surgery. Nonoperative therapy is usually reserved for patients who are not candidates for surgery. The goal of therapy for these patients is palliation of dysphagia. Chemotherapy, radiotherapy, or both are used to relieve dysphagia for these patients, to kill possible residual cancer cells in postoperative cases, and to limit the cancer in patients with advanced esophageal cancer as preoperative treatment.
Chinese medicinal herbs have commonly been used as an adjunct treatment for alleviating the side effects of chemotherapy or radiotherapy and for improving the quality of life of esophageal cancer patients. Possible benefits of herbal medicines include increasing appetite, boosting the immune system, facilitating the recovery of the body, and preventing tumor regeneration or the development of metastases. A Cochrane systematic review including two trials suggested that Zhenxiang capsules or Huachansu injection may not improve the effects of short-term therapy or the 1-year survival rate when used as an adjunct to chemo- or radiotherapy in the treatment of esophageal cancer. The quality of life may be improved by Huachansu injection; however, the evidence was weak due to the poor quality of these two studies. The purpose of this article was to review publications to assess the efficacy and possible adverse effects of Chinese medicinal herbs integrated with chemo- or radiotherapy for the treatment of esophageal cancer.
Methods
Criteria for Considering Studies for this Review
Randomized controlled trials referring to the clinical utility and safety of the administration of traditional Chinese medicines (TCM) in any form, for example, oral decoction, intravenous, or gastrogavage, for the treatment of esophageal cancer as an adjunct to active cancer therapy such as radiotherapy or chemotherapy were considered as eligible for inclusion. The comparator could be active therapy without TCM or placebo. Patients with different subtypes at any stage of esophageal cancer were eligible for inclusion.
Outcomes
Outcomes reviewed included the following:
Mortality or median survival times
Time to progression
Quality of life, include reducing gastrointestinal tract symptoms and other adverse reactions caused by radiochemotherapy
Improvement of disease, defined as a complete response and partial response as clarified by Miller, or short-term therapeutic effects
Immune function as defined by T-lymph cell subsets
Adverse events, defined as any adverse event resulting from TCM that caused death, life-threatening illness, or significant toxicity
Search Methods for Identification of Studies
We searched electronic databases using the terms esophageal cancer and Chinese herbs or traditional Chinese medicine or Chinese medicinal herbs . Trials were identified by searching the following electronic databases up to September of 2008: the CENTRAL database of the Cochrane Library (issue 2, 2008), MEDLINE, the Chinese Biomedical Database, the China National Knowledge Infrastructure, the VIP database, and the WANFANG database.
Data Collection and Analysis
The study selection was undertaken by two reviewers (YXZ and WTX).
Selection of Studies
Full articles of studies that mentioned “randomly allocated patients” were retrieved for further assessment. At first, the authors of potential included studies were telephoned to determine whether they were randomized controlled trials. There were no recorded disagreements between reviewers.
Data Extraction
Data extracted from each of the included studies concerned the details of the study population, the type and stage of esophageal cancer, the duration and dosing schedule of interventions, outcomes, and any treatment-related adverse events observed. Methods and quality issues were also extracted (eg, the design, duration of follow-up, method of randomization, allocation concealment, and blinding).
Assessment of Potential Risk of Bias of Included Studies
Several characteristics were assessed according to the quality standard previously described by Wu and Liu.
Randomization process
A. A random number table or computer software used to generate random numbers was judged as an adequate procedure of randomization. Coin tossing or shuffling used for generating the random allocation sequence before the trial launching was considered eligible and associated with a low risk of selection bias.
B. A study that did not specify one of the adequate methods outlined previously but only mentioned “random” was considered to have a moderate risk of selection bias.
C. Other methods of allocation (eg, quasi-randomization) or that optionally allocated the patients that appeared to have a high risk of bias were excluded.
Allocation concealment process
A. Measures to conceal allocation were defined as adequate when the person who generated an allocation sequence did not intend to recruit the participants (eg, central randomization) and when the allocation sequences were conserved safely by special persons (eg, sealed in opaque envelopes or kept in a secured computer) or another description was used that contained convincing elements of concealment. These measures were considered to have a low risk of selection bias.
B. Concealed trials in which the author did not report an approach of allocation concealment at all were considered as having a moderate risk of selection bias.
C. Inadequate allocation concealment was defined as an approach that did not fall into one of the categories in A.
D. The study did not conceal allocation.
Methods C and D were considered as having a high risk of selection bias.
Level of blinding
A. In double or triple blinding, the health care provider, participants, and results assessor were masked. These studies were considered as having a low risk of performance and detection bias.
B. Single blinding of the results assessor was considered to have a moderate risk of performance and detection bias. If single blinding of the patients but not of the results assessor was performed, the study was considered to have a high risk of detection bias.
C. Nonblinding was considered to have a high risk of performance and detection bias.
There was no bias for assessment of mortality whether blinded or not.
Incomplete outcome data
A. There was a low risk of bias in trials in which few drop-out/losses to follow-up were noted and an intention-to-treat analysis was possible.
B. There was a moderate risk of bias in trials reporting that the rate of exclusion was about 10%, regardless of whether intention-to-treat analysis was used.
C. There was a high risk of bias when the rate of exclusion was at least 15%, or when wide differences in exclusions between groups occurred, regardless of whether intention-to-treat analysis was used.
Selective report
No. There was a low risk of reporting bias. All of the outcomes were reported in detail.
Probably yes. There was a moderate risk of reporting bias. At least one of outcomes was mentioned but not in detail.
Yes. There was a high risk of reporting bias. At least one of the outcomes was not reported.
Measures of Treatment Effects
Due to the differences in the formulas used in the included studies, we did not perform combined analysis. The effects of dichotomous data were measured as relative risk (RR) with a 95% confidence interval (CI). Continuous data as were presented as a mean difference (MD) with a 95% CI.
Results
Description of Studies
Results of the search
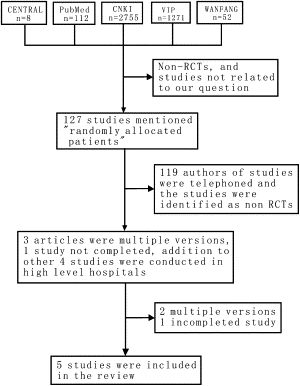
The initial search of the electronic databases yielded 8 studies in CENTRAL, 112 in PubMed, 2755 in the Chinese National Knowledge Infrastructure, 1271 in VIP, and 53 in WANFANG. After scrutinizing these studies, 127 studies mentioning “randomly allocated patients” were identified. All of these studies were conducted in China. A total of 119 authors of these studies were contacted by telephone, and the studies were identified as nonrandomized controlled trials and therefore excluded. One study obtained outcomes that did not match our inclusion criteria and was also excluded.
The author of a published study that included 40 patients was communicated with by telephone. The author stated that the study currently included 100 patients and that the data were being analyzed. We considered this to be an incomplete study and decided to classify it as an ongoing study.
One study was duplicated three times. We decided to abstract data from the first version. The authors of these three reports and other authors of four studies could not be contacted by telephone to confirm the randomization method used and other quality issues. Nevertheless, we included these studies in the review because they were performed in high-level hospitals and could be representative of TCM used in the treatment of esophageal cancer. The flowchart of included studies is shown in Fig. 1 .
Characteristics of included studies
Design
Four studies had two parallel arms and one had three. “Randomly allocated patients” were mentioned.
Studied populations
A total of 406 esophageal cancer patients were included from five studies. Two studies included postoperative patients; the others included advanced esophageal cancer patients.
Interventions
In three studies, chemo- or radiotherapy or both were given to both groups. In addition to chemo- or radiotherapy, TCM decoction was given to TCM group patients orally. The contents of TCM decoctions were modified according to different TCM signs. The modified traditional TCM formula Sijunzi Tang decoction, Liuwei Dihuan Wan decoction, and self-prepared formula were adjunctively used during the radio- or chemotherapy, respectively.
In one study, one group received standard chemotherapy, one group received a TCM decoction prepared by authors themselves according to the signs of patients for 12 months, and one group received both chemotherapy and an oral TCM decoction for 12 months. The contents of TCM preparations are listed in Table 1 .
| Preparation | Pinyin Name | Latin Name | English Name |
|---|---|---|---|
| Liuwei Dihuang Wan | |||
| Shu Dihuang | Radix rehmanniae | Rehmannia root | |
| Shanzhuyu | Fructus corni | Common macrocarpium fruit | |
| Shanyao | Rhizoma diosscoreae | Common yam rhizome | |
| Mudanpi | Cortex moutan | Tree peony bark | |
| Fuling | Poria | Indian buead | |
| Zexie | Rhizoma alismatis | Oriental waterplantain rhizoma | |
| Sijunzi Tang decoction | |||
| Renshen | Radix ginseng | Ginseng | |
| Fuling | Poria | Indian buead | |
| Baizhu | Rhizoma atractylodis macrocephalae | Largehead atractylodes rhizome | |
| Gancao | Radix glycyrrhizae | Liquoric root | |
Results
Description of Studies
Results of the search
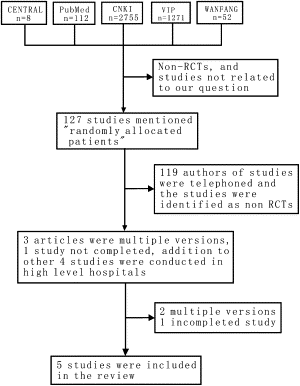
The initial search of the electronic databases yielded 8 studies in CENTRAL, 112 in PubMed, 2755 in the Chinese National Knowledge Infrastructure, 1271 in VIP, and 53 in WANFANG. After scrutinizing these studies, 127 studies mentioning “randomly allocated patients” were identified. All of these studies were conducted in China. A total of 119 authors of these studies were contacted by telephone, and the studies were identified as nonrandomized controlled trials and therefore excluded. One study obtained outcomes that did not match our inclusion criteria and was also excluded.
The author of a published study that included 40 patients was communicated with by telephone. The author stated that the study currently included 100 patients and that the data were being analyzed. We considered this to be an incomplete study and decided to classify it as an ongoing study.
One study was duplicated three times. We decided to abstract data from the first version. The authors of these three reports and other authors of four studies could not be contacted by telephone to confirm the randomization method used and other quality issues. Nevertheless, we included these studies in the review because they were performed in high-level hospitals and could be representative of TCM used in the treatment of esophageal cancer. The flowchart of included studies is shown in Fig. 1 .