Fig. 12.1
The correct plane in total mesorectal excision
TME is associated with improved local control and survival rates. The local recurrence rate following total mesenteric excision with an APR or sphincter-sparing procedure ranges from 4 to 7 % [7–9] This remains an improvement when compared to the local recurrence rates following the conventional blunt approach which ranged from 14 to 45 %, with or without postoperative radiation therapy (RT) or chemoradiotherapy [10].
Rationale for TME and Its Relationship to Anatomy of Spread of Rectal Cancer
The lymphatic and venous drainage of the rectum are cephalad and lateral (Figs. 12.2 and 12.3). The upper two thirds of the rectum drains along the pathway of the superior hemorrhoidal vein, cephalad to the inferior mesenteric nodes, and the para-aortic nodes. The lymphatic drainage of the lower third of the rectum is cephalad as well as laterally along the middle hemorrhoidal vessels to the internal iliac nodes. There are no communications between the inferior mesenteric and internal iliac lymphatics [11]. In women, lymphatic drainage above the dentate line also includes the posterior wall of the vagina and reproductive organs. Below the dentate line, the drainage is along the inferior rectal lymphatics to the superior inguinal nodes and along the pathway of the inferior rectal artery [12].
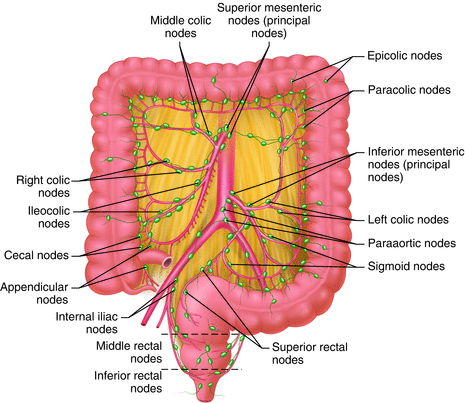
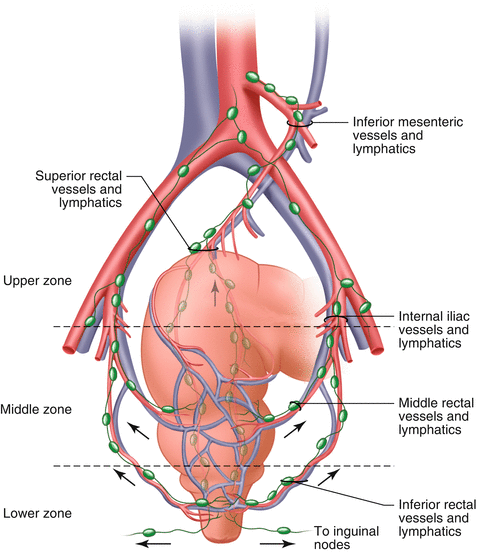

Fig. 12.2
The lymphatic drainage of the colon and rectum

Fig. 12.3
Blood supply to the rectum and the accompanying lymphatic and venous drainage. Showing the cephalad drainage of upper rectum and lateral pathways from the middle rectum
It is believed that the improved local recurrence rates with TME is the result of improved lateral clearance and circumferential margin negativity, and removal of potential tumor deposits in the mesentery as well as a decreased risk of tumor spillage from a disrupted mesentery [13]. Improved local control appears to result in better survival [14].
Genitourinary Complications of Pelvic Dissection
TME preserves the pelvic autonomic nerves which reduces the risk of postoperative genitourinary dysfunction [10].
Urinary dysfunction:
Urinary dysfunction after proctocolectomy, primarily manifested as difficulty voiding, is thought to be the result of autonomic nerve injury leading to impairments in parasympathetic innervation to the detrusor muscle and/or sympathetic innervation to the bladder neck, trigone, and urethra. Urodynamic studies reveal a significant postoperative decrease in effective bladder capacity and increases in first sensation to void and residual urinary volume compared with the preoperative evaluation [15]. Incidence of urinary dysfunction has been reported to be 30–60 %, with the greatest risk following abdominoperineal resection [16]. Urinary dysfunction persisting beyond the early (30-day) postoperative period has been reported in 12 % of patients [17].
Autonomic sparing procedures can be effectively performed when dissecting the pelvis. A prospective study of 20 patients undergoing a total mesorectal excision (TME) with an autonomic nerve preservation (ANP) technique and sphincter preservation found no significant difference between preoperative and postoperative mean residual volume after micturition [18].
Sexual dysfunction:
Sexual dysfunction following proctocolectomy is related to the extensiveness of the dissection of the pelvic nerves and occurs in both men and women.
In men, damage to the sympathetic nerves during high ligation of the inferior mesenteric artery or posterior dissection at the sacral promontory can lead to retrograde ejaculation, and damage to the parasympathetic plexus (nervi erigentes) during lateral and anterior dissection can lead to erectile dysfunction.
Female infertility
The majority of data regarding female infertility may be extrapolated from data specific to inflammatory bowel disease (IBD).
Pelvic surgery is common in patients with inflammatory bowel disease and the majority of these patients are child bearing age. The infertility rate before pelvic surgery for such women is less than 10 % which is similar to that of the general population, however, the rate of infertility following a restorative proctocolectomy is significantly increased to 26–48 % [21, 22].
The cause of infertility is thought to be mechanical from scarring of fallopian tubes.
With the recent increase in incidence of rectal cancer in younger populations, women should be counseled preoperatively regarding the risk of infertility.
Pelvic Autonomic Nerve Anatomy
Surgeons are familiar with visceral and vascular anatomy of the pelvis, however, neuroanatomy is less apparent and its importance is not appreciated.
The parasympathetic nervous system is referred to as the craniosacral outflow; the pelvic splanchnic nerves comprise the sacral outflow component (Fig. 12.4). They arise from the ventral rami of the S2–S4 and enter the sacral plexus. They travel with the inferior hypogastric plexus, located bilaterally on the lateral walls of the pelvis. From there, they contribute to the innervation of the pelvic and genital organs. They regulate the emptying of the urinary bladder and the rectum as well as sexual function, including erection (Fig. 12.5).
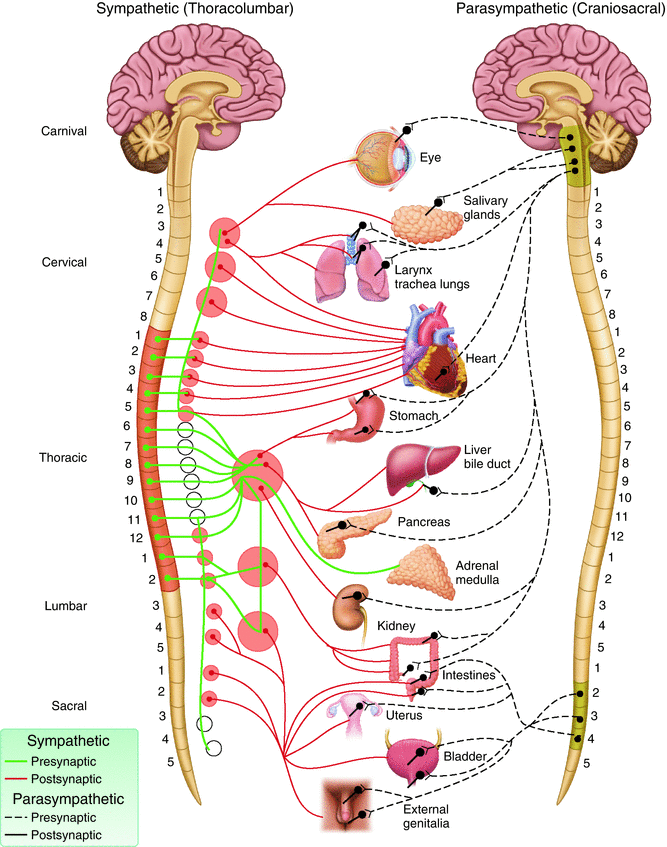
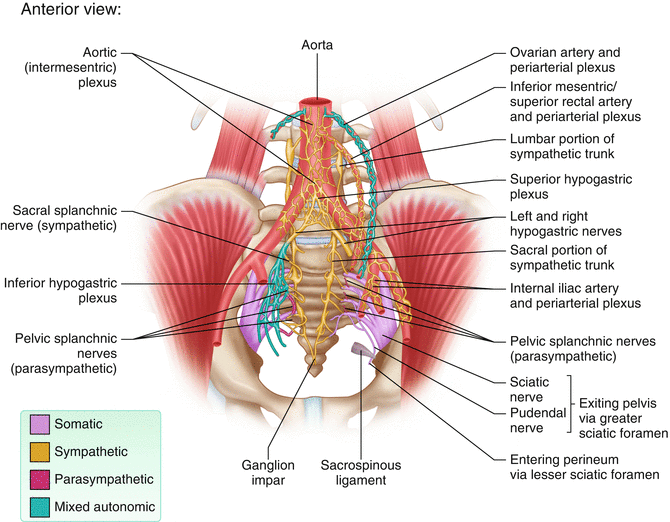

Fig. 12.4
Distribution of autonomic nervous system

Fig. 12.5
Connections of sacral and pelvic splanchnic nerves
Sacral splanchnic nerves, which arise from the sympathetic trunk, provide sympathetic efferent fibers (Fig. 12.4). The superior hypogastric plexus is a ganglionic plexus that lies over the bifurcation of the aorta in the presacral space. From there, the nerves split into two hypogastric nerves that run along the internal iliac vessels. These nerves connect to the inferior hypogastric plexus on the pelvic sidewall (Fig. 12.6).
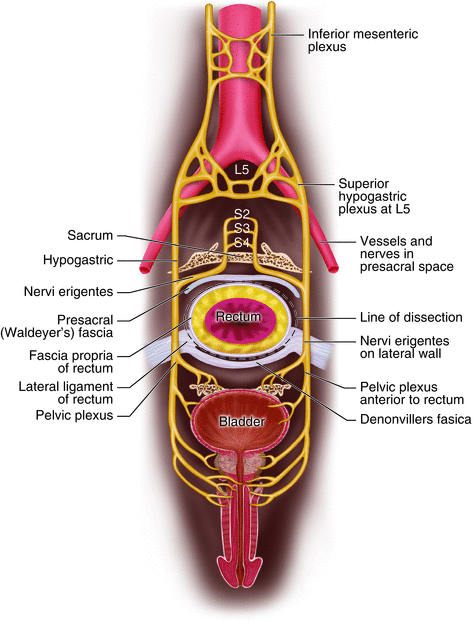

Fig. 12.6
Mesorectal plane of excision in relation to the pelvic plexus
The “lateral ligament of the rectum” (LLR) has long been the subject of anatomical confusion and surgical misconception. Some surgeons believe that the lateral ligaments are artifacts produced by the obsolete process of blunt dissection during rectal mobilization [23]. Lin and colleagues performed cadaveric dissection on 32 cadavers to elucidate the anatomy of this structure [24] and report that the constant component of the lateral ligament of the rectum included the rectal branches from the pelvic plexus, whereas the middle rectal artery was not apparent in the majority of lateral ligaments. Additionally, they concluded that the LLR was located traversing between the rectum and visceral fascia, thus making it difficult to reveal the LLR if performing TME within the correct surgical plane (between visceral fascia and parietal fascia) (Fig. 12.7).
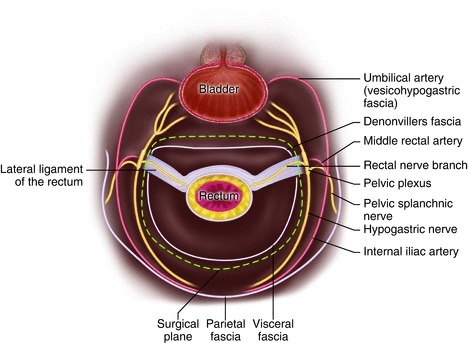

Fig. 12.7
Relationship of surgical plane with the lateral ligament
Technical Aspects of TME and ANP
The goals of a curative resection for rectal cancer include performing a wide resection of the cancer, therefore achieving histologically negative margins as well as performing a total mesorectal excision (TME) including resection of local lymph nodes.
When performing TME the following points should be kept in mind
1.
Complete removal of the mesorectum, including the lateral and circumferential margins of the mesorectal envelope [10].
2.
Removal of the inferior mesenteric artery (IMA) and the locoregional lymphatic system of the rectum.
3.
The use of sharp, rather than blunt dissection, in the avascular plane between the parietal and visceral pelvic fascia [25]. Conventional blunt dissection violates the mesorectal circumference and potentially leads to substantial hemorrhage thus leaving residual pelvic tumor.
In regards to autonomic nerve preservation, care must be taken not to damage the pelvic plexus during lateral dissection of the lateral ligament along the pelvic sidewall. Enker [26] describes important aspects of autonomic nerve preservation technique. In addition to sharp dissection along the parietal pelvic fascia and maintaining intactness of mesorectal envelope, it is important to preserve the superior hypogastric plexus where it lies on the anterior surface of the aorta and divides into the two hypogastric nerves running along each side of the pelvis to join the inferior hypogastric plexus and the pelvic splanchnic nerves from S 2–4. For all resections, the anterior plane of dissection is immediately anterior to the fascia of Denonvilliers’ in men and immediately posterior to the vagina in women. Prior to completion of the operation, the nerves should be inspected and checked for any damage.
Intraoperative Nerve Monitoring
In efforts to improve nerve sparing surgery, intraoperative electrical stimulation of pelvic autonomic nerves has been proposed in urology and gynecology [27]. Lue et al., first reported the successful use of intraoperative electrical nerve stimulation to monitor the function of cavernous nerves in 16 patients undergoing radical retropubic prostatectomy [28]. Kneiste and colleagues performed intraoperative nerve stimulation (INS) in 62 patients to confirm pelvic autonomic nerve preservation (PANP). They compared nerve stimulation to visual assessment by the surgeon and found that INS results in higher sensitivity (82 vs 46 %). In addition, the accuracy of INS in predicting PANP was higher (88 vs 83 %) and the correlation between urinary function and the findings on INS was better (kappa-value: 0.65 vs 0.40). A prospective, randomized, single-blinded, multi-center trial is underway with an expected completion date of December 2015. This study will examine the impact of continuous monitoring for preservation of urogenital function in patients with TME for rectal cancer. 188 patients will be included in two arms: TME with and without intraoperative continuous monitoring of pelvic autonomic nerves. (ClinicalTrials.gov Identifier: NCT01585727)
Laparoscopic TME
In experienced hands, mesorectal excision can be done with comparable results to open surgery [29] with the advantages of less postoperative ileus and pain, and a shorter length of hospital stay when compared to open surgery [30]. Additionally, possible benefits of minimally invasive surgery include better visualization and magnification, and lack of blunt dissection.
In terms of sexual and bladder dysfunction after laparoscopic surgery there is limited data showing its superiority. A retrospective review of patients from a previous randomized trial comparing outcomes of laparoscopic with open resection of rectal cancer found an increased risk of sexual dysfunction in those undergoing a laparoscopic compared with an open resection in men who were previously sexually active (7 of 15 versus 1 of 22 patients). There was no difference for sexual function for women. There was no difference in rates of bladder dysfunction [31]. It should be noted that the same principles of mesorectal excision with preservation of autonomic nerves applies to the laparoscopic approach.
The technical aspects of the laparoscopic approach to nerve sparing proctectomy with mesorectal excision for rectal cancer can be found at the following link to the American Gastrointestinal and Endoscopic Surgeons (SAGES) video library: http://www.sages.org/video/laparoscopic-nerve-sparing-proctectomy-with-mesorectal-excision-for-rectal-cancer-2.
Robotic TME
Robotic rectal surgery is an emerging technology that combines the advantages of the laparoscopic approach, including reduction in postoperative pain and faster recovery, with high-quality three-dimensional vision [32]. Based upon small retrospective reviews, robotic-assisted total mesorectal excision is safe and feasible with no difference in number of harvested lymph nodes or circumferential resection margins compared with open and laparoscopic approaches [33–35]. In a case-matched analysis of patients undergoing robotic-assisted, laparoscopic-assisted, or an open approach for the resection of mid- or low rectal cancer (165 patients in each arm), no significant difference in 2-year disease-free survival was identified [36].
Lymphadenectomy and Vascular Ligation
The goal of total mesorectal excision is to harvest the complete nodal basin for staging purposes, local control, and the interruption of the metastatic process. The higher number of lymph nodes harvested, the more accurate the staging. A 12 lymph node benchmark was adopted as a quality metric by the American College of Surgeons, the National Comprehensive Cancer Network (NCCN), and the American Association of Clinical Oncology (ASCO) [37]. However, recent studies have shown that the number of harvested lymph nodes is affected by pre-operative chemoradiation [38]. It is believed that the removal of the draining lymph nodes to the level of the proximal vascular pedicle, rather than the absolute number of lymph nodes, is an important surgical principle. For colon cancer the use of a lymph node ratio (the ratio of metastatic to examined lymph nodes) has been suggested as a means of incorporating both the number of involved nodes and the total number examined into prognostic stratification [39].
The level of vascular ligation is a surrogate for resection of the nodal basin. Some authors suggest removal of the blood supply and lymphatics up to the level of the origin of the superior rectal artery, which is just caudal to the takeoff of the left colic artery (low tie) [40]. When the inferior mesenteric artery (IMA) is ligated at its origin (high tie), lymph node yield may be increased, however, no significant difference in survival has been found between the two techniques [41]. It should be noted that when nodes above the superior rectal drainage are clinically suspicious, the resection should be extended proximally to include high ligation of the IMA. In addition, high ligation of the IMA at the origin at the aorta will likely provide better mobilization for a tension-free coloanal anastomosis. On the other hand the height of vascular ligation can theoretically put nerve integrity at jeopardy at the aorta.
Extended Lateral Lymphadenectomy
Lateral lymph node dissection (LLND) includes removal of all nodal tissue along the common and internal iliac arteries. Some studies have shown improved local recurrence and survival [42]. A meta-analysis comparing LLND with conventional surgery found that LLND did not confer a significant oncological benefit, but it was associated with increased urinary and sexual dysfunction [43]. Therefore, an extended lateral lymph node dissection is not necessary in the absence of clinically negative nodes in this region. Additionally, in a recent study from Washington University patients with enlarged lymph nodes in the lateral region on staging CT scan had no increase in local recurrence when treated with neoadjuvant chemoradiation therapy and no extended lateral lymphadenectomy [44].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






