Benefits
Drawbacks
Earlier control of electrolyte/metabolic derangement
Iatrogenic episodes of hemodynamic instability that may impede kidney repair and recovery
Earlier control of acid–base derangement
Insertion of dialysis catheter and risk of catheter-associated complication (i.e., bleeding, thrombosis, bloodstream infection, and pneumothorax)
Avoidance and earlier control of complications of uremia
Uncertain clearance of micronutrients, trace elements and sub-therapeutic levels of vital medications (i.e., antimicrobials, anti-epileptics)
Earlier management of fluid status and avoidance of excessive fluid accumulation and overload
Unnecessary exposure to RRT in those who will spontaneously recover kidney function with conservative management
Avoidance of unnecessary diuretic exposure
Need for immobilization
Potentially beneficial immunomodulation
Use of health resources and increased health care costs
On the other hand, there is no robust high quality evidence to support the practice that earlier initiation of RRT, in the absence of a life-threatening complication of AKI, impacts important patient centered outcomes such as renal recovery or survival. These perceived benefits of RRT have to naturally be balanced with the potential harm attributable to RRT, including risks associated with iatrogenic episodes of hemodynamic instability, central venous insertion of a dialysis catheter, exposure of blood to an extracorporeal circuit, need for anticoagulation of the extracorporeal circuit, uncertain medication clearance (i.e., antimicrobials) and unwanted depletion of micronutrients. In addition, there is a possibility that with a more conservative strategy of supportive management and watchful waiting, and initiation of RRT only when a life-threatening complication develops, some patients with severe AKI may indeed recover kidney function spontaneously [19]. As a result, early RRT in some patients may unnecessarily expose patients to the risks of RRT and result in less favorable outcomes, unnecessary bedside resources and incremental costs [20].
12.2 Triggers for Starting RRT
When considering whether to initiate RRT, most clinicians make this decision based on the following clinical, physiologic and laboratory factors and their trajectories: serum creatinine, and urea including the presence of uremic complications, serum potassium, acid–base status, urine output, fluid balance, overall course and prognosis of the patient’s illness, and the patient’s preferences for escalation of life-sustaining therapy with RRT [21]. Among these triggers, some are considered absolute indications to avert potentially life threatening complications and others are considered more relative (Table 12.2). Recently, the issue of fluid balance, accumulation and/or overload has received focused attention as a potential modifiable factor associated with outcome and has emerged as a determinant for considering RRT [22–24]. To know whether there is a role for the application of routine RRT primarily for immunomodulation to remove inflammatory mediators such as in sepsis is the focus of ongoing investigations [25].
Table 12.2
Summary of absolute and relative indications for starting RRT in critically ill patients with AKI
Absolute indications | In the absence of contraindications or limitations of organ support, indications for urgent/emergency RRT include: |
Refractory, rapidly rising, or cardiac toxicity associated hyperkalemia (K > 6.5 mmol/L) | |
Refractory metabolic acidosis (pH ≤7.2 despite normal or low arterial pCO2) | |
Refractory pulmonary or non-renal organ edema unresponsive to diuretic therapy | |
Symptoms or complications attributable to uremia (i.e., pericarditis, encephalopathy, and coagulopathy) | |
Overdose/toxicity from a dialyzable drug/toxin | |
Relative indications | In the absence of life threatening complications of AKI, important factors that might influence the decision to start RRT include: |
Limited physiological reserve to tolerate the consequences of AKI (i.e., pre-morbid advanced CKD) | |
Advanced non-renal organ dysfunction intolerant to excessive fluid accumulation (i.e., impaired cardiac function) | |
Anticipated solute burden (i.e., tumor lysis syndrome; rhabdomyolysis; and intravascular hemolysis) | |
Need for large fluid administration (i.e., nutritional support, medications, or blood products) | |
Severity of the underlying disease (affecting the likelihood of recovery of kidney function) | |
Concomitant accumulation of poisons or toxic drugs which can be removed by RRT (i.e., salicylates, ethylene glycol, methanol, and metformin) |
12.3 Literature Review
The optimal timing for RRT remains unclear [26, 27]. Very few randomized clinical trials and numerous observational studies of variable methodological rigor have evaluated the issue of timing of RRT initiation in critically ill patients with AKI [16–18]. These studies vary widely in their criteria for defining “early” and “late” RRT, often using arbitrary cut-offs for serum creatinine, serum urea or urine output, fluid balance, time from ICU admission or duration of AKI. This has created challenges for making clear inferences to inform clinical practice.
In a pilot trial, Bouman et al randomized 106 critically ill predominantly cardiac surgical patients with oliguric AKI despite fluid resuscitation, inotropic support and diuretic therapy, to a strategy of early versus late initiation of RRT [28]. The early group started RRT within 12 h of fulfilling eligibility, defined by oliguria (<30 ml/h for 6 h and no response to a diuretic challenge or hemodynamic optimization), or a creatinine clearance <20 ml/min. The late group started RRT when classic indications were fulfilled including a serum urea >40 mmol/L, potassium of >6.5 mmol/L or evidence of pulmonary edema. In this study, there were no differences in survival, recovery of kidney function or health resource utilization beyond RRT. However, this trial was not adequately designed to assess these outcomes; was not viewed as widely generalizable due to an unexpectedly high observed survival and a large number of patients who had cardiac surgery-associated AKI. Notably, six patients allocated to the late group did not start RRT (four due to renal recovery; and two due to death) and of those who started RRT, 50 % had developed fluid overload and pulmonary edema. In a small single-centre trial from India, 208 hospitalized patients with community-acquired AKI were randomized to either (1) early RRT, characterized by starting RRT after serum urea exceeded 23 mmol/L or serum creatinine exceeded 618 μmol/L irrespective of other AKI complications, or (2) standard of care where RRT was only initiated in the setting of medically-refractory hyperkalemia, acidosis or volume overload or in the setting of uremic symptoms [29]. In this study, there were no observed differences in mortality or recovery of kidney function. This trial also has limited generalizability due to the young demographics of enrolled patients (mean age 42 years), the predominant aetiology of AKI (>50 % tropical infections or obstetric complications), and due to most patients not being critically ill.
Several single-centre controlled trials in cardiac surgery patients have suggested that earlier RRT, most often defined as initiation within 8 h of surgery, can reduce morbidity, improve survival and reduce overall post-operative resource use [30–35]. The concluding inference from these small non-randomized trials is that early initiation of RRT for patients with AKI following cardiac surgery should be triggered by a worsening oliguria rather than actual serum creatinine results.
Several observational studies have also evaluated the optimal timing of RRT for critically ill patients with AKI, as summarized in recent systematic reviews [16–18]. While these studies have numerous methodological limitations, low quality, and high risk of bias, the majority have suggested that “earlier” initiation of RRT was associated with improved outcomes [36–41].
In a secondary analysis of the multinational Beginning and Ending Supportive Therapy (BEST) for the Kidney cohort study, the timing of initiation of RRT was evaluated in 1,238 critically ill patients with AKI [42]. Late RRT, defined relative to time from ICU admission (≥5 days) was associated with higher adjusted-mortality (OR, 1.95; 95 % CI, 1.30–2.92; p = 0.001). Furthermore, the duration of RRT and hospitalization, and the rate of RRT dependence at hospital discharge, were greater when the interval from ICU admission to RRT initiation was prolonged. Other studies have shown similar results [20, 36]. In a multi-centre prospective Canadian study, the characteristics of critically ill patients with AKI at the time RRT was initiated, were evaluated [43]. At RRT initiation, serum creatinine and urea were 331 (225–446) μmol/L and 22.9 (13.9–32.9) mmol/L, respectively. Oligo-anuria (<400 mL/24 h) was present in 32.9 %, and 92.2 % had a positive fluid balance. Notably, only 16.2 % had hyperkalemia (serum potassium ≥5.5 mmol/L) and 33.8 % had metabolic acidosis (serum bicarbonate ≤15 mmol/L) at RRT initiation. These data highlight that the decision to initiate RRT was often influenced by numerous patient-specific factors and that the majority (>80 %) had two or more recognized triggers; however, this study also found that the occurrence of life threatening urgent indications for RRT initiation was relatively infrequent in the ICU. In a secondary analysis of 239 critically ill patients with severe AKI treated with RRT in the FINNAKI study, the impact of the presence of classic indications for RRT on 90-day all-cause mortality were evaluated [44]. The primary exposure was the timing of starting RRT relative to evidence of developing one or more “conventional” indications for RRT which included hyperkalemia, severe acidemia, uremia, oligo-anuria and severe fluid overload with pulmonary edema. Timing was classified as “pre-emptive” if RRT was started in the absence of these criteria; “classic – urgent” if started within 12 h of developing one of these indications; and “classic – delayed” when started more than 12 h after developing one of these indications. In multivariable and propensity-adjusted analyses, pre-emptive RRT was associated with lower 90-day mortality compared with RRT after a classic indication developed (30 % vs. 49 %; odds ratio [OR] 2.1; 95 % CI 1.0–4.1). Ninety-day mortality was also markedly lower among patients having “classic – urgent” RRT compared with when RRT was delayed (39 % vs. 68 %; OR 3.9; 95 % CI 1.5–10.2). Moreover, mortality among patients with pre-emptive RRT was found lower compared to those with AKI not treated with RRT in an adjusted propensity-matched analysis.
12.4 Current Clinical Practice Guideline Recommendations
Since 2012, the Kidney Disease Improving Global Outcomes (KDIGO) consortium and the National Institute for Health and Care Excellence (NICE) in the United Kingdom have published official recommendations related to the timing of RRT [26, 27].
The KDIGO Clinical Practice Guideline (CPG) for AKI acknowledged that both the ideal indication and the optimal timing for initiation of RRT in patients with AKI were uncertain, [26] and accordingly, by consensus, KDIGO provided the following recommendations:
(i)
Initiate RRT emergently when life-threatening changes in fluid, electrolyte, and acid–base balance exist (Sect. 5.1.1 – Not Graded).
(ii)
Consider the broader clinical context, the presence of conditions that can be modified with RRT, and trends of laboratory tests—rather than single BUN and creatinine thresholds alone—when making the decision to start RRT (Sect. 5.1.2 – Not Graded)
The KDIGO CPG clearly recognizes that there is a paucity of a strong evidence base for these recommendations and suggests that clinicians assess not only the presence of life-threatening complications when considering RRT, but also the wider clinical status of the patient, including the underlying trajectory of illness severity, burden of non-renal organ dysfunction and the expectation of whether complications attributable to AKI will arise.
Similarly, the NICE CPG for AKI, based on the findings from two randomized trials and three prospective observational studies, made the following recommendations pertaining to initiation of RRT [27]:
(i)
Discuss any potential indications for renal replacement therapy with a nephrologist, pediatric nephrologist and/or critical care specialist immediately to ensure that the therapy is started as soon as needed.
(ii)
Refer adults, children and young people immediately for RRT if any of the following are not responding to medical management:
Hyperkalemia
Metabolic acidosis
Complications of uremia (i.e., pericarditis or encephalopathy)
Fluid overload
Pulmonary edema
(iii)
Base the decision to start RRT on the condition of the adult, child or young person as a whole and not on an isolated urea, creatinine or potassium value.
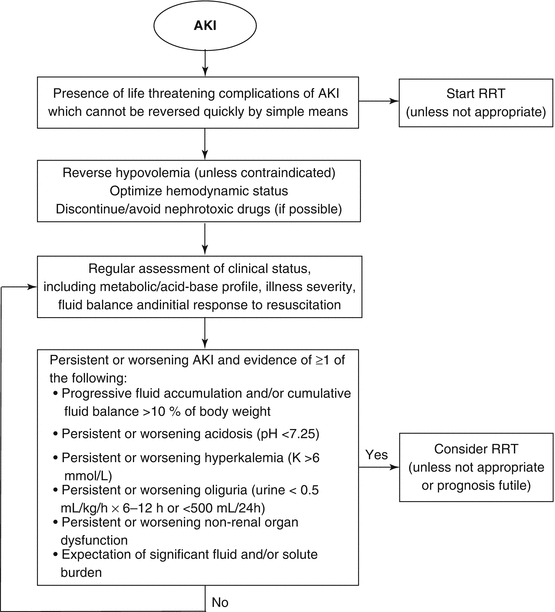
The NICE recommendations also highlight the lack of evidence to support when to optimally start RRT. Moreover, NICE emphasizes that better tools are needed to identify those patients with AKI who are less likely to recover renal function with a conservative strategy alone and need a period of renal support, and patients in whom RRT can be safely avoided. It is possible that some of the newly discovered biomarkers for AKI will fulfil this role. Figure 12.1 gives some guidance for clinical management and decision making at the bedside [21].
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






