Chapter 2 Thoracoscopic Lung Resections
Positioning and placement of trocars
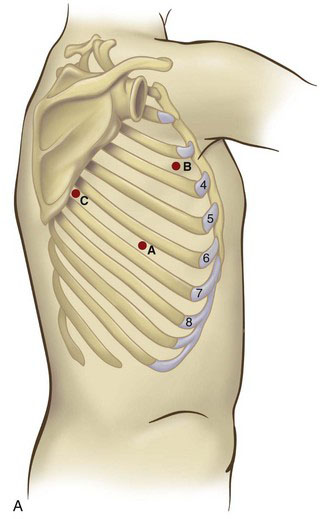
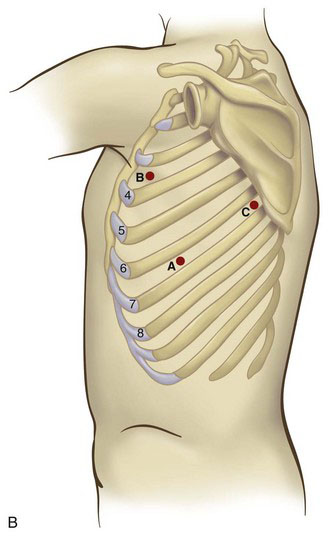
With the patient in the lateral decubitus position (Fig. 2-1), the initial port site is in the seventh or eighth intercostal space at the midaxillary line (Fig. 2-2). This port will be used for the camera in most cases; ideally, the port will be just above the level of the diaphragm. This corresponds to point A in Figure 2-2. In general, the greater the body mass index (BMI) of the patient, the higher the level of the diaphragm; this requires cephalad movement of the camera site so that the diaphragm does not impair thoracoscopic visualization. The diaphragmatic position can be determined by reviewing the preoperative chest radiograph. This port site should be created under direct visualization to avoid passing through the intercostal space and diaphragm simultaneously, which would result in intra-abdominal camera placement.
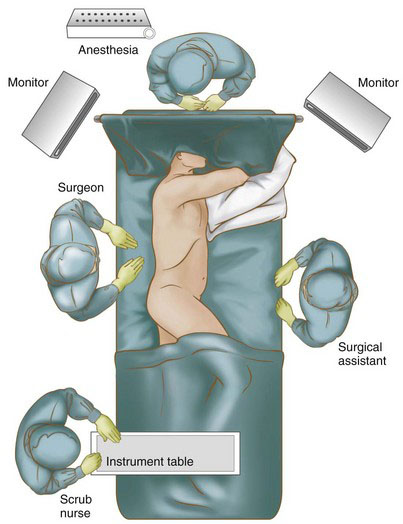
FIGURE 2-1 Patient and operating team positioning for a VATS lobectomy (right-sided procedure shown).
Before placing the camera port, digital examination of the pleural space should be performed to assess for the presence of adhesions or even pleural tumor implants. Many of these adhesions can be cleared by digital sweeping, but denser adhesions may need sharp dissection. Once there is an adequate space to insert the thoracoscope, the pleural space can undergo further evaluation. Because there is no need for insufflation, a simple reusable port typically is sufficient. A 30-degree scope provides excellent visualization of the upper mediastinum, the subcarinal area, the diaphragm, and the pericardium. Two additional port sites are placed under direct vision. The anterior port site (point B in Fig. 2-2) is placed in the fourth intercostal space when an upper lobectomy is planned, whereas the fifth intercostal space is used for a middle or lower lobectomy. The third (posterior) port is placed in the seventh intercostal space, anterior to the scapular edge (point C in Fig. 2-2). Unsuspected pleural metastases can be resected for biopsy if they are identified, and any adhesions are lysed at this time.
Operative technique
Right Upper Lobectomy
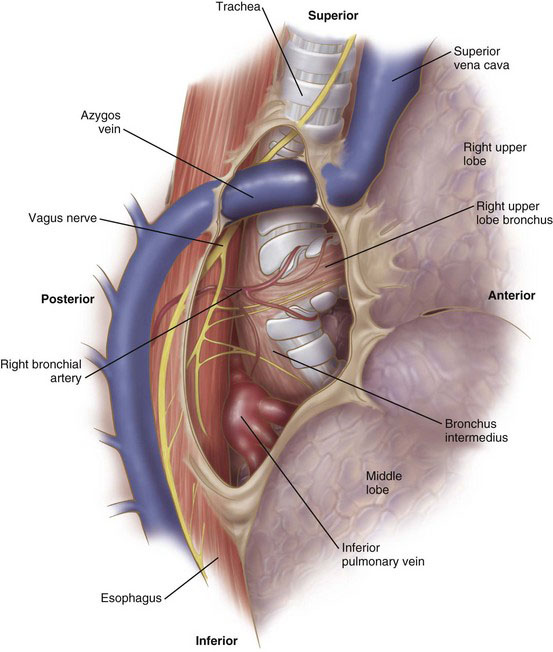
After placement of the ports, the lung is retracted anteriorly. The pleura along the posterior aspect of the hilum is opened with the electrocautery device. With gentle blunt dissection, the confluence of the right upper lobe bronchus and the bronchus intermedius is identified (Fig. 2-3). There usually is a lymph node located at this bifurcation. Clearing this area at the beginning of the operation will expedite completion of the fissure later in the procedure. The lung then is retracted posteriorly. The pleura on the anterior aspect of the hilum is opened with the cautery. The location of the phrenic nerve needs to be monitored at all times during the dissection of the anterior hilum. Clearing this portion of the pleura will identify the trunks of the superior pulmonary vein, which drain the right upper and right middle lobes. This dissection also will demarcate the fissure between the right upper lobe and right middle lobe. In some patients, a branch of the right middle lobe vein crosses the fissure and drains into the posterior segment vein. Whenever possible, this crossing branch should be spared, taking the upper lobe vein proximal to this branch.
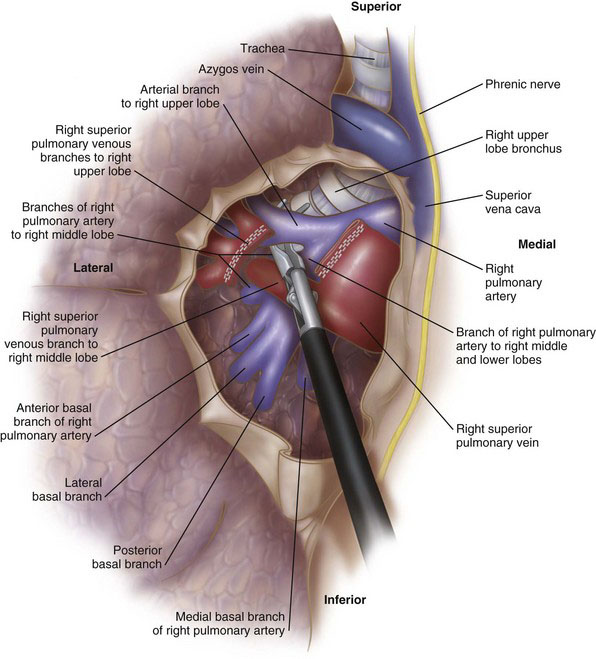
The upper lobe vein is isolated by a combination of blunt and sharp dissection (Fig. 2-4). A large, blunt right-angle clamp can be used to clear the soft tissue behind the vein. This must be done carefully because the pulmonary artery is located immediately behind the vein. The vein then is divided with the vascular stapler. One option for positioning the stapler is to place a red rubber catheter on the stapling device to guide the blade behind the vein. Placement of the stapler blade behind the vein also can be facilitated with the use of a large right-angle clamp.
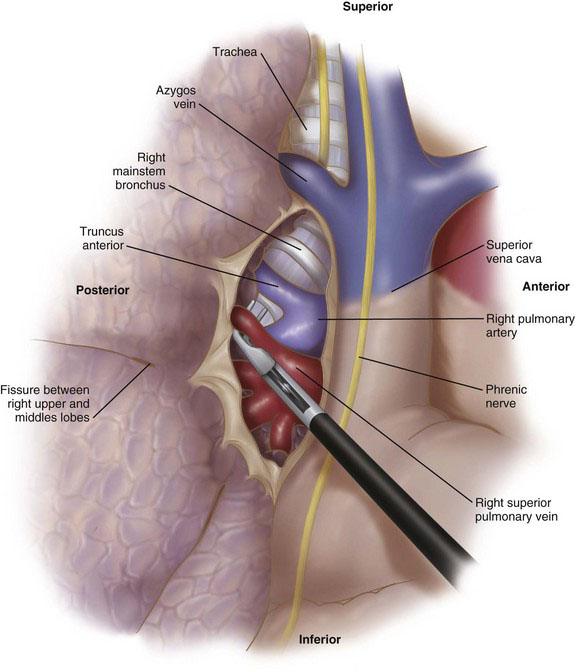
FIGURE 2-4 Exposure of the anterior hilum, with dissection of the right superior pulmonary vein (VATS RUL).
Division of the vein exposes the right pulmonary artery (Fig. 2-5). There usually is a single large arterial branch that supplies the upper lobe, although occasionally there will be two to three smaller branches. The right upper lobe pulmonary artery branch, once cleared, is divided with the stapling device. At this point there usually are two remaining structures to be divided: (1) the branch of the pulmonary artery supplying the posterior segment of the right upper lobe, and (2) the bronchus. It often is easier to clear the bronchus and divide this structure before stapling the posterior segment branch of the pulmonary artery (Fig. 2-6). Alternatively, depending on how complete the fissure is between the right upper and lower lobes, the remaining arterial branch can be divided first, followed by the bronchus. It is imperative to ensure correct placement of the stapler across the upper lobe bronchus by ventilating the middle and lower lobes before firing the stapler. After division of all hilar structures, the fissures can be completed with a laparoscopic stapler-cutter (e.g., Endo GIA, Covidien, Norwalk, Conn).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree