Fig. 13.1
Colonic anastomotic stricture: endoscopic aspect
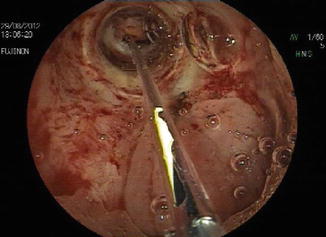
Fig. 13.2
Colonic anastomotic stricture: endoscopic dilation with TTS balloon
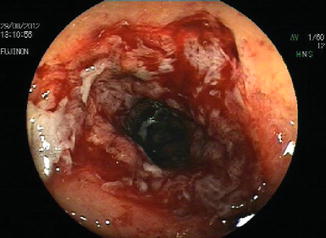
Fig. 13.3
Colonic anastomotic stricture: endoscopic aspect after dilation
13.4 Stenting
Stenting therapy usually represents the last attempt before surgery in cases of refractory stenosis and is therefore the recommended treatment after 5 or 6 ineffective dilation sessions. Absolute contraindications are esophageal strictures located less than 2 cm from the superior esophageal sphincter and rectal strictures located less than 5 cm from the anal sphincter [13]. Stent types include partially covered SEMS and fully covered SEMS and fully covered SEPS and BD stents . In benign diseases stents are not intended to be permanent and safe removal is an essential feature [14]. The technical success rate of stent placement ranges from 85 to 100 % [14, 15]. Dilation prior to stenting should be avoided (high risk of perforation), and it is not recommended to cross the stent with the endoscope at the end of the procedure (high risk of device dislocation). Endoscopic follow-up after stent placement is not well defined: some authors perform endoscopy after 4–6 weeks or earlier if complications occur, while others propose surveillance with endoscopy or radiology every 2 weeks, with stent removal in the event of stricture [14, 15]. The optimal duration of this treatment has not yet been established, and recommendations are based on data from small case series. Some authors report that 4–8 weeks after placement is the optimal time for removal [16]; during extraction reactive tissue in- and overgrowth can determine major and minor adverse events which subsequently require surgery [17]. Metal stents, long stenting duration, large-diameter devices, and high-expansion force are all associated with high rates of reactive tissue; this problem is infrequent with BD stents, and thus the development of new covered BD stents could further lower the rate of tissue ingrowth [18, 19]. Stent migration is mainly related to device features but is also linked to stenosis peculiarities, and short anastomotic strictures are stent migration-promoting factors. Attempts to prevent stent migration by clipping the device to the intestinal wall have shown poor results; in the large bowel, covered stents almost uniformly migrate even when fixation techniques are used, and so no dedicated covered colonic stents are currently commercially available in the USA [13]. Dilation or placement of a new stent after device explantation is sometimes necessary (up to 21 % in some studies); in cases of ineffective endoscopic treatment surgery is required (up to 50 % in some case series) [14]. Stent placement provides immediate symptom relief and may obviate the need for surgery in selected patients, but data on long-term patency and safety of this technique are lacking [14].
Metal stents . The main limitation of SEMS is tissue neoformation that causes recurrent symptoms in >15 % of patients, a high rate of complications, such as perforation and obstruction, and precludes easy stent removal; although hyperplastic tissue reaction is lower with covered stents, it has also been reported to occur with these devices [14]. With regard to tissue reaction, partially covered SEMS show low migration (overall 12 %), can be difficult and traumatic to remove, and should be avoided because of the high risk of complications (recurrent stricture formation and mucosal damage, bleeding, or perforation after removal); conversely, fully covered SEMS are easy to remove but have a higher migration rate than the former. SEMS are thus not recommended or approved for benign strictures by the US Food and Drug Administration [15]. Some recent studies have examined new coated or temperature-sensitive nitinol stents which show encouraging technical and clinical results and are easy to remove [16].
Plastic stents . SEPS were recently introduced to reduce the risk of hyperplastic tissue growth associated with metal stents. They are effective and safe compared to the metal devices and can be easily removed, but migration occurs more frequently, often when the stricture improves [18]. Polyflex stent (Boston Scientific Corp., Natick, Massachusetts, USA), a self-expanding plastic polyester silicone-coated stent, is particularly effective because the radial expansive force is similar to that of SEMS and the silicone coating resists tissue ingrowth and hyperplasia [18]. The Polyflex esophageal stent is currently the only FDA-approved retrievable stent marketed in the USA. Esophageal stenosis such as that found in refractory benign strictures is one of the FDA-approved indications for Polyflex stents [18]. SEPS stenting time is not well defined: although some authors consider 6 weeks as the optimal duration (X), a longer period probably results in a higher success rate in cases of refractory strictures. It has therefore been hypothesized that the optimal stenting time depends on stricture features. The role of SEPS is still widely debated because of conflicting results from various studies, with success rates ranging from 0 to 94 % and an unexpected high rate of complications described in some cases series but not in others (in the majority of studies SEPS appear to be relatively safe) [20].
Biodegradable stents . BD stents would appear to eliminate the risk of mucosal hyperplastic reaction, have a shorter patency, show low migration, and do not require subsequent removal: the prolonged dilatory effect before absorption and the progressive degradation could represent a favorable solution for refractory anastomotic strictures (stent degradation is about 3–4 months and the radial force remains around initial values for the first 5 weeks, decreases to about 2/3 of the initial force after 7 weeks and further reduces to about 1/2 of the starting force at week 9) [21].
These stents often need an anastomotic stricture pre-dilation of up to 12–20 mm to facilitate the introduction of the delivery system and to ensure adequate device expansion; however, pre-dilation increases the risk of stent migration. Several authors advise endoscopy and fluoroscopy to be repeated at 1-month intervals in order to monitor stent position, patency, and degradation; in some studies clinical and radiographic follow-up is performed 1 week after insertion and again 1 month later, whereas endoscopic evaluation is only performed in the presence of symptoms [7]. Although mucosal hyperplasia after BD stenting can cause reobstruction, this problem responds well to single-balloon dilation and resolves completely after stent degradation [7]. Anastomotic stricture recurrence after complete stent degradation is easily resolved, treatment based on balloon dilation of up to 18–20 mm (usually only one session is needed to obtain an adequate lumen size) or on a new BD stent placement [22]. The main disadvantages of BD stents are that they can only be placed in areas close to the mouth or the anus [23], the devices tendency to migrate if they are not fixed to the wall, and that a second stent after degradation in more long-standing strictures may become necessary. Even if anastomotic stricture long-term patency after BD stents placement is better than after dilation, there is a lack of published series; probably a longer BD stent duration would be associated with a higher long–term success rate, and consequently new devices with delayed degradation process could well give a more prolonged benefit. The efficacy of steroid injections in improving the effects of BD stents warrants investigation [24, 25].
13.5 Incisional Therapy, Argon Plasma Coagulation, Intralesional Steroid Injection
There are no standard recommendations for the management of persistent or recurrent strictures resistant to dilation, and thus various techniques have been adopted: incisional therapy and Nd:YAG laser/APC, with or without dilation, intralesional steroid injection combined with dilation, stenting, and finally endoscopic surgery [6]. Incisional therapy , performed using a needle knife or polypectomy snare and evaluated in small cases series, is safe and particularly effective in short strictures (<10 mm). In one study it was used in patients previously treated with dilation therapy or not and showed a success rate of 87.5 % after only one session [26]. This treatment has been successfully used together with balloon dilation or APC in uncontrolled studies, showing a good safety profile [6]. A combination of APC and Savary dilation appears to be potentially useful in strictures that do not respond after three sessions of mechanical dilation [26]. In one study on 10 patients, neodymium: yttrium–aluminum–garnet laser used in association with balloon dilation determined technical and clinical success without recurrence or complications in 90 % of patients with a median follow-up of 82 months. In various small, uncontrolled studies, some authors treated recurrent anastomotic strictures with steroid injection followed by dilation (intralesional steroid injections increase the effect of dilation by reducing collagen formation through the local inhibition of the inflammatory response) [27]; steroid treatment combined with dilation has an uncertain benefit for the initial treatment of benign strictures [27].
13.6 Endoscopic Treatment in Different Anastomotic Sites
13.6.1 Esophagus
Anastomotic strictures after esophageal resection occur in 5–46 % of patients; although there is no universal consensus, an esophageal anastomosis is normally considered stenotic when the diameter is less than 12 mm. Proximal strictures are particularly difficult to treat and are at a higher risk of complications [26]. The success rate of dilation therapy ranges from 70 to 90 %, but 40 % of patients require more than 3 dilation sessions; the risk of relapse can be reduced by performing a dilation of 18–20 mm, but a diameter of 12 mm may be sufficient to mitigate dysphagia to solids [26]. Intralesional steroid injections associated with balloon dilations increase the maximum dilation diameter achieved and reduce the severity of symptoms (significant improvement in the dysphagia score: 0.63 ± 0.59 vs 2.42 ± 0.5, P < 0.001), the number of dilation sessions required, and the rate of recurrence [27]. The clinical success rate of fully covered SEMS is less than 50 % [28, 29]; a recent meta-analysis highlighted an improvement in dysphagia in 46 % of patients with benign strictures after placement of various removable stents, and another pooled analysis with Polyflex stent placement showed a clinical success rate of 52 % [18, 28]. In the literature more than 15 papers have reported promising results for Polyflex stents placed for benign esophageal disorders: technical success 96 % (range 75–100 %), clinical success 89 % (range 69–100 %), and migration 27 % (range 7–57 %) [18]. A number of studies have tempered initial enthusiasm regarding SEPS by reporting high stent migration (62.1 %), a low rate of long-term improvement after stent removal (17 %), the need for new stent placement (55 %), and dysphagia relapse (70 %) [18]. A recent review evaluated a total of 10 studies on 130 patients with benign esophageal strictures treated with SEPS placement (49 anastomotic strictures, 39 %): technical success 98 %, clinical success 52 %, early migration with fully covered stents 24 %, need for endoscopic reinterventions 21 %, and major complications 9 % [18]. Only a small number of studies have focused on the use of BD stents , reporting complete relief from dysphagia in 40–60 % of cases [19]; however, BD stents are “Conformité Européenne” marked, certified to conform to European regulations for the treatment of patients with refractory benign esophageal stricture. One study investigated the efficacy and safety of a BD stent (Ella-BD stent, Ella-CS, s.r.o., Hradec Kralove, Czech Republic) in 21 patients with refractory benign esophageal strictures, showing that this treatment is safe and effective: technical success 100 %, stent migration 9.5 %, recurrent dysphagia caused by stent obstruction secondary to hyperplastic tissue in- and overgrowth 5 %, clinical success after a follow-up of 6 months 45 %, and major complications 0 % [29]. Another recent study evaluated safety and long-term efficacy of the same stent in 28 patients with refractory benign esophageal stricture previously treated with multiple dilations associated or not with nonbiodegradable stenting; total technical success was 93 % and 13 patients were treated with sequential BD stent placement (median 3, range 2–8) showing that sequential stenting, in selected cases, may be a valid option to avoid serial dilations or surgery [30]. In cases of anastomotic strictures located near the upper esophageal sphincter, the use of stents has been associated with an extremely high rate of complications (almost 100 %), suggesting that dilation or incisional therapy may be better alternatives. Some esophageal stenoses are particularly complex making it very difficult to identify the true lumen of the anastomosis; in such cases an endoscopic “rendezvous” technique, i.e., combined antegrade and retrograde dilation, can be applied through a gastrostomy or jejunostomy to reduce the risk of a false route.
13.6.2 Stomach and Small Bowel
Anastomotic strictures associated with bariatric surgery are now the most frequent indications for endoscopic treatment (estimated rate of postoperative stricture formation following gastroplasty is about 2 %, while that for Roux-en-Y gastric bypass [RYGB] is around 3–27 %) [31]. The most common region of anastomotic stricture is the site of gastrojejunostomy, and the mean time to diagnosis after surgery is generally 3 months [31]. Gastrojejunostomy should have a diameter of 10–12 mm: in case of higher diameter surgical treatment executed is useless, while in case of lesser diameter problems of canalization may arise. Endoscopic stricture control is achieved in 95–100 % of cases; the best endoscopic technique remains to be defined, but pneumatic dilation is the most widely used method, and many endoscopists prefer to use TTS balloons, dilating them to at least 15 mm in the first session in order to decrease the risk of recurrence [32]. As the optimal diameter of the pylorus is approximately 12 mm, balloons with a smaller diameter, e.g., such as 10, 11, or 12 mm, may be sufficient [32]. Pneumatic dilation is an effective and safe treatment strategy with a high overall success rate; the number of dilations required to successfully treat a gastrojejunal stricture is unknown, but many reviews have reported that the majority of patients require at least two dilation sessions [32–34]. Balloon dilation can produce desirable results in the short term (symptom improvement following the initial dilation is observed in 58–93 % of patients), but further research is needed to identify the endoscopic treatment modality that results in long-term success [33, 34]. Savary dilation is also effective and safe: 100 % clinical success has been achieved using a maximum diameter of 12.8 mm and performing endoscopies 1–2 weeks after each session, the majority of patients requiring only one (46 %) or two dilations (50 %) and not experiencing complications [35]. Mechanical dilation may be useful in case of pneumatic failure; using Savary with size of 15–18 mm in anastomotic strictures treated unsuccessfully with initial pneumatic dilation, clinical success is 100 % and complication rate is low (1.6 %) [33]. Diathermy combined with APC has been used in limited cases with good results (only one treatment session to gain long-term recanalization in 92 % of patients) [26]. The use of stenting in post-RYGB anastomotic strictures is not particularly frequent or useful because of the high stent migration rate (about 60 % of cases) whose occurrence is related to stenosis length and small bowel peristalsis [35]. A study has evaluated the clinical efficacy and safety of balloon dilation and stent placement in 63 patients undergoing treatment for early benign anastomotic strictures after gastric surgery: clinical success was achieved in 89 % of patients after a single-balloon dilation (49 %), multiple-balloon dilations (32 %), and stent placement (8 %), highlighting that pneumatic dilation is safe and effective and that stenting can be effective in selected refractory patients [35]. The development of double–balloon endoscopy has rendered possible the endoscopic therapy of anastomoses located in sites which in the past could only be treated by surgeons; various endoscopic treatments are currently possible for the small intestine, and stent placement is also feasible, depending on the lesion site [36, 37]. As the small intestine wall is very thin, great care must be taken during endoscopic therapy to avoid complications such as bleeding and perforation. Although endoscopic balloon dilation may be taken into consideration as the first therapeutic option, a single session is generally not sufficient and symptoms may recur after treatment [38]. Dilation diameter is determined on the basis of stricture size, and the first dilation is usually performed up to a diameter of 12 mm (this diameter allows intake of a low-residue diet); sometimes, with a higher perforation risk, it is possible to dilate up to 15 mm. In cases of a tight stricture, dilation should initially be limited to a small diameter and gradually increased in size in multiple therapeutic sessions [39].
13.6.3 Large Bowel
Colonic postoperative stricture, which usually occurs from 1 to 9 months after surgery in 5.8–30 % of cases, is more common after colorectal anastomosis and in cases of intraperitoneal stapled anastomoses rather than extraperitoneal ones [3]. Stricture occurs more frequently in cases of previous neoplastic resection (p < 0.05), and anastomoses of previous oncologic surgery are more difficult to treat than those of benign resection (TTS balloons, technical success: benign resection 88–92.8 %, oncologic surgery 59–61.5 %) [2, 8]. A colonic anastomosis is generally defined as stenotic when it is not transitable by a 13 mm endoscope [2, 3]. Endoscopic dilation should be considered as the first therapeutic approach as it is immediately effective and repeatable and does not preclude surgery [2, 5]. Balloon dilation is the treatment of choice, with clinical success rates ranging from 86 to 97 % (usually 2 or 3 dilations are needed for a good long-term result) [2, 8]. Treatment end point is the easy transit of a standard colonoscope through the stricture after dilation. Ileocolic and colic anastomotic strictures can be dilated up to 15–20 mm without specific risks and sometimes, if the 20 mm diameter is easily reached, it is also possible to use a 30 mm balloon. In active Crohn’s disease, long and complex anastomotic strictures with marked inflammation should not be dilated due to a high risk of perforation (11 %). When proximal to the anus, anastomotic strictures can be treated not only with TTS balloons but also with Savary dilators, Eder–Puestow metal olives, and achalasia balloons [40]. Treatment with Savary is clinically successful or partially successful in 80 % of cases; the majority of patients requiring 1–3 dilation sessions performed 14–21 days after the previous endoscopy [9]. Eder–Puestow metal olives can be used in rectal anastomoses; abundant scar tissue surrounding the stenosis could explain the low perforation rate [9]. Achalasia balloons show a 94–100 % clinical success in patients and have a low complication rate (0–11 %); the mean number of dilations ranges from 1 (29 %) to 4.5 (24 %), and the clinical success decreases from the short (87.2 %) to the long term (66.6 %) [2]. Several removable stents specifically designed for use in the colon are currently available [41]. SEMS show a technical success of over 90 % and a clinical success from 63 to 91 %. Covered stents with diameters of less than 25 mm are associated with a high risk of migration, and this complication, associated to obstruction, may limit the role of these devices in benign colonic strictures. Although plastic colonic stents are effective, there is still insufficient evidence for it to be recommended for use in clinical practice [1]. Two studies that included the highest number of patients treated with covered SEMS or SEPS to date showed a technical success of 95–96 %, clinical success of 50–63 %, migration in 21.4–32 %, and major complications in 0–25 % [10]. Treatment with BD stents is feasible, and high migration rates can be resolved by appropriate improvements in the design of devices [7]. An important problem in the placement of these stents is the required proximity of the anastomosis to the anus because the positioner is relatively inflexible, and it may not be possible to place a stent more than 30 cm from the anus [7]. The role of stenting in the management of refractory ileocolonic anastomotic strictures after resection for Crohn’s disease is widely debated with limited data and widely disparate outcomes. A recent review reported that stenting can provide lasting benefit in select patients: technical success 100 %, clinical success 80 %, mean long-term luminal patency 34.8 months, and reobstruction rate 20 % with surgical intervention [42]. Another study evaluated the role of BD stents in patients with stenosing Crohn’s disease of the small and/or large intestine: technical success 90 %, early stent migration 27 %, clinical success after a follow-up of 16 months 63.6 %, and major complications 0 % [42]. A higher number of stent options are available for rectosigmoid anastomoses, including the use of esophageal stents: in such patients treatment is possible with TTS and non-TTS stents. Treatment with stents in rectal anastomotic strictures is limited by the required distance of the stricture from the anal verge in order to avoid painful impingement of the device upon the sphincter muscles and by the rigid nature of the delivery devices. Colorectal anastomosis may occasionally close completely, and a variety of endoscopic techniques have been proposed to resolve the problem [43]. In these cases it is possible to use a suprapapillary biliary puncture catheter inserted into the center of the anastomosis; a 0.025 in. guidewire is passed through the catheter into the colon, and balloon dilation is performed up to a diameter of 2 cm. Although this treatment is useful and safe, it should only be performed by skilled endoscopists [43].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








