Therapeutic apheresis (TA) refers to a group of extracorporeal procedures in which blood separation technology is used to remove abnormal blood cells and/or plasma constituents. The terms plasmapheresis, leukapheresis, erythrocytapheresis, and thrombocytapheresis describe the specific blood element that is removed. In plasmapheresis, or therapeutic plasma exchange (TPE), large quantities of plasma are removed from a patient and replaced with fresh frozen plasma (FFP), or albumin solutions in normal saline.
I. RATIONALE FOR PLASMAPHERESIS (TPE). There are several mechanisms by which plasmapheresis exerts its beneficial effects (Table 18.1). Its major mode of action is rapid depletion of specific disease-associated factors. Another effect is its ability to remove other high-molecular-weight proteins that may participate in the inflammatory process (intact complement C3, C4, activated complement products, fibrinogen, and cytokines). Several other theoretical effects of TPE on immune function have been proposed, including immunomodulatory actions such as alterations in idiotypic/anti-idiotypic antibody balance, a shift in the antibody-to-antigen ratio to more soluble forms of immune complexes (facilitating their clearance), and stimulation of lymphocyte clones to enhance cytotoxic therapy. TPE also allows the infusion of normal plasma, which may replace a deficient plasma component, perhaps the principal mechanism of action of TPE in thrombotic thrombocytopenic purpura (TTP).
A. Principles of treatment
1. Use of concomitant immunosuppression. Because of the immunologic nature of most diseases treated by plasmapheresis, therapy should almost always include concomitant immunosuppression. Adjunct medication protocols usually include high doses of corticosteroids, cytotoxic drugs, and biologic agents. These medications are expected to reduce the rate of resynthesis of pathologic antibodies and to further modulate cell-mediated immunity, which may contribute to many of these disorders.
2. Early treatment. Diseases that respond to plasmapheresis are best treated early to halt the inflammatory response that often contributes to disease progression. For example, plasmapheresis of anti–glomerular basement membrane (GBM) disease is most effective if therapy is initiated when serum creatinine is <5 mg/dL (440 mcmol/L).
Possible Mechanisms of Action of Therapeutic Plasma Exchange | |
Removal of Abnormal Circulating Factor
Antibody (anti-GBM disease, myasthenia gravis, Guillain–Barré syndrome)
Monoclonal protein (Waldenström macroglobulinemia, myeloma protein)
Circulating immune complexes (cryoglobulinemia, SLE)
Alloantibody (Rh alloimmunization in pregnancy)
Toxic factor
Replenishment of Specific Plasma Factor
TTP
Other Effects on the Immune System
Improvement in function of reticuloendothelial system
Removal of inflammatory mediators (cytokines, complement)
Shift in antibody-to-antigen ratio, resulting in more soluble forms of immune complexes
Effects on the cellular immune system
GBM, glomerular basement membrane; SLE, systemic lupus erythematosus; TTP, thrombotic thrombocytopenic purpura.
II. PHARMACOKINETICS OF IMMUNOGLOBULIN (IG) REMOVAL
A. Plasma half-life. Immunoglobulins have relatively long half-lives, approaching 21 days for IgG and 5 days for IgM. Because of the relatively long plasma half-lives of the immunoglobulins, the use of immunosuppressive agents that decrease their production rate cannot be expected to lower the plasma levels of a pathogenic autoantibody for at least several weeks, even if production is completely blocked. This is the basic rationale for their removal by extracorporeal means.
B. Extravascular distribution and equilibration rate. Immunoglobulins have a substantial extravascular distribution (Table 18.2). The extent of intravascular versus extravascular distribution will determine how effectively they can be removed in the course of a single plasmapheresis session. Immunoglobulins exhibit an intravascular-to-extravascular equilibration that is approximately 1%–2% per hour, whereas extravascular-to-intravascular equilibration may be somewhat faster because it is governed by the rate of lymphatic flow. Still, since the extravascular-to-intravascular equilibration is relatively slow, the kinetics of immunoglobulin removal by plasma exchange can be calculated by using first-order kinetics governing removal rates from a single compartment (the intravascular space).
Distribution Volumes of Immunoglobulins | |
C. The macromolecule reduction ratio and Ve/Vp. In Chapter 3, the relationship between the urea reduction ratio (URR) and Kt/V was described. A similar relationship holds for removal of immunoglobulins by TPE.
The kinetics of immunoglobulin removal by TPE follows an exponential relationship:
![]()
where C0 = the initial plasma concentration of the macromolecule in question, Ct = its concentration at time t, Ve = the volume of plasma exchanged at time t, and Vp = the estimated plasma volume, which, while smaller than the volume of distribution of many of these macromolecules, functions as the volume from which they are removed, given the slow rate of equilibration between the extravascular and intravascular compartments.
The macromolecule reduction ratio (MRR), expressed as a percentage, is 100 × (1 − Ct/C0), so MRR = 100 × (1−e−Ve/Vp). If we plug in numbers for Ve from 1,400 mL to 8,400 mL (Table 18.3), and if we assume that a patient’s Vp is 2,800 mL, we will get values of Ve/Vp from 0.5 to 3.0. TPE using these Ve/Vp ratios will result in values for the MRR (Table 18.3) ranging from 39% (when Ve/Vp = 0.5) to 95% (when Ve/Vp = 3.0). Note that for Ve/Vp = 1.0, the MRR is 63%. The largest decrease (MRR) occurs with removal of the first plasma volume; removal of subsequent plasma volumes during the same session becomes progressively less effective in decreasing the concentration of the macromolecule in question. The effectiveness of the procedure after one plasma volume is further reduced because of the dilution of the substance to be removed by the exchange fluid. For this reason, usually 1.0–1.5 plasma volume equivalents (Ve/Vp) are exchanged during a plasmapheresis session.
Relationship between Plasma Volume Removed and Concentration of Substance | |
Portion of Plasma Volumea Exchanged (Ve/Vp) | Volume Exchanged (Ve, mL) | Immunoglobulin or Other Substance Removed (MRR, %) |
0.5 | 1,400 | 39 |
1.0 | 2,800 | 63 |
1.5 | 4,200 | 78 |
2.0 | 5,600 | 86 |
2.5 | 7,000 | 92 |
3.0 | 8,400 | 95 |
Ve, volume of plasma exchanged; Vp, estimated plasma volume; MRR, macromolecule reduction ratio.
aPlasma volume = 2,800 mL in a 70-kg patient, assuming hematocrit = 45%.
D. Reaccumulation. Subsequent to the removal of the macromolecule in question, there is a reaccumulation of its concentration in the vascular space from two sources: redistribution and further synthesis. Redistribution from the extravascular space occurs via lymphatic drainage into the vascular space, as well as from diffusion of the macromolecule across capillaries from the interstitial to the intravascular space. Endogenous synthesis has been documented in Goodpasture syndrome, in which the anti-GBM antibodies will be predictably lowered by a given plasma exchange treatment, but intertreatment increases in serum levels are too rapid to be compatible with simple re-equilibration from extravascular stores.
E. Pharmacokinetic basis for TPE prescriptions. Based on these concepts, a rational approach to prescribing TPE is generally to recommend one plasma volume exchange daily or every other day, depending on the disease process, to allow time for adequate redistribution of macromolecules via lymphatic drainage into the vascular space. The rate of accumulation and the frequency of TPE should be targeted to the specific macromolecule that is pathogenic, if this is known. For example, whereas the half-life of IgG is approximately 21 days, that of IgM and IgA is much shorter (5–7 days). Therefore, if the macromolecule in question is IgM, there may be a role for a more extended period of TPE because the endogenous synthesis rate is expected to be higher for IgM than for IgG. In addition, the distribution of IgM is predominantly intravascular, while the distribution of IgG is mainly in the extravascular space. Therefore, when removing IgM antibodies or paraproteins, daily TPE is warranted. On the other hand, patients with presumed IgG autoantibodies should be treated every other day to allow for IgG redistribution from the extravascular space into the intravascular compartment. If the substance to be removed is measurable by reliable quantitative means (such as with specific autoantibody), then the treatment schedule should be designed to achieve a significant reduction of that substance based on kinetic considerations. If treatments are performed without identification of the offending agent, then the physician remains dependent on empirical treatment regimens.
F. Estimation of plasma volume. An estimate of the plasma volume is required to arrive at an appropriate plasmapheresis prescription. For this purpose, there are several nomograms and equations using height, weight, and hematocrit (Hct). These have been incorporated into newer versions of plasmapheresis equipment. A useful rule of thumb is to consider plasma volume to be approximately 35–40 mL/kg of lean body weight, with the lower number (35 mL/kg) applicable to patients with normal Hct values and 40 mL/kg applicable to patients with Hct values that are less than normal. For example, in a 70-kg patient with a normal Hct (45%), plasma volume (Vp) would be 70 × 40 = 2,800 mL.
Predicted blood volume equations have been derived by curve-fitting techniques using subjects’ height (cm) and body weight (kg) compared with actual blood volumes measured by isotope (iodine-131 albumin) dilution techniques: Vp = (1 − Hct) (b + cW), where W = lean body weight, b = 1,530 for males, 864 for females, and c = 41 for males, 47.2 for females. It is important to remember that these calculations are based on lean body weight. Therefore, for obese patients one must use lean body mass to avoid unnecessary and dangerously large volume exchanges.
III. TECHNICAL CONSIDERATIONS. TPE can be performed using centrifugation blood cell separators or by membrane plasma separation (MPS). Centrifugation devices are commonly used for blood banking since they are capable of selective cell removal (cytapheresis) in addition to plasmapheresis. MPS utilizes highly permeable hollow-fiber filters, similar to dialyzers but with large pore sizes and appropriately modified dialysis equipment. The advantages and disadvantages of each technique are summarized in Table 18.4.
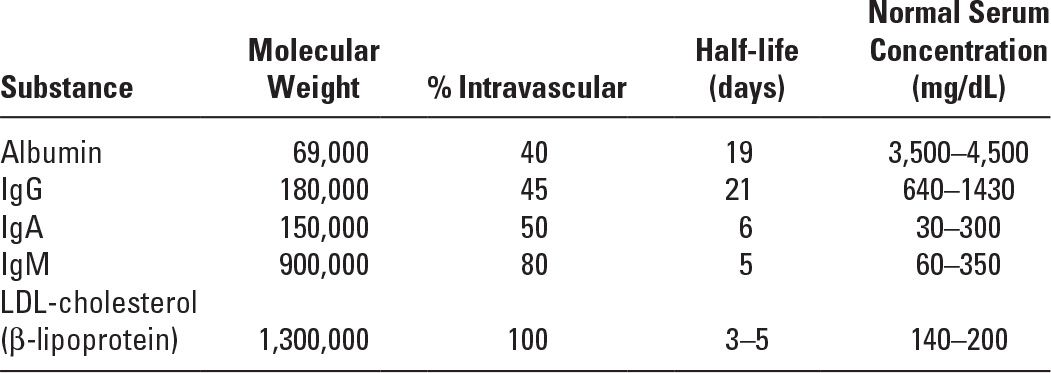
A. Centrifugal apheresis. During centrifugation, blood cells are separated by gravity, based on the different densities of the blood components. There are two centrifugation methods used in blood cell separators: intermittent-flow (or discontinuous-flow) devices and continuous-flow devices. Red blood cells (RBCs) move to the outside of the spinning container, while plasma, the lightest component, remains on the inside. Platelets and white blood cells (WBCs) localize between the red cell and plasma layers. Any of these components can be collected, discarded, or reinfused (Fig. 18.1).
Comparison of Membrane Plasma Separation and Centrifugal Apheresis | |
| Advantages | Disadvantages |
Membrane plasma separation | Faster and smaller equipment | Removal of substances limited by sieving coefficient of membrane |
|
| Reduced efficiency in hyperviscosity syndromes and cryoglobulinemia |
| No citrate requirements | Unable to perform cytapheresis |
| Can be adapted for cascade filtration | Requires high blood flows, central venous access |
|
| Requires heparin anticoagulation, limiting use in bleeding disorders |
Centrifugal apheresis | Capable of performing cytapheresis | Large and heavy equipment |
| No heparin requirement | Requires citrate anticoagulation |
| More efficient removal of all plasma components | Loss of platelets |
In the intermittent-flow separation devices, multiple aliquots of blood are sequentially withdrawn and routed to a bowl, where each aliquot is processed and then reinfused. In the continuous-flow method, blood is withdrawn, centrifuged, and separated, and the desired component removed or returned to the patient in a continuous mode using a hoop-shaped annulus that has strategically placed sampling ports (Fig. 18.1) for the collection of plasma, RBCs, WBCs, and platelets. The intermittent-flow method requires a single-needle vascular access, while the continuous-flow system requires two venous accesses (one for withdrawal and a second one for return) or a dual-lumen dialysis-type venous catheter. Intermittent-flow blood cell separators (Haemonetics Corporation, Braintree, MA) are rarely used today for therapeutic apheresis. The continuous-flow devices are preferred for therapeutic procedures because of their smaller extracorporeal blood volume, significantly shorter procedure time, and lesser anticoagulant requirement. The most widely used centrifugal blood cell separators for therapeutic apheresis are made by Terumo BCT (Lakewood, CO) and Fresenius Kabi (Bad Homburg, Germany).
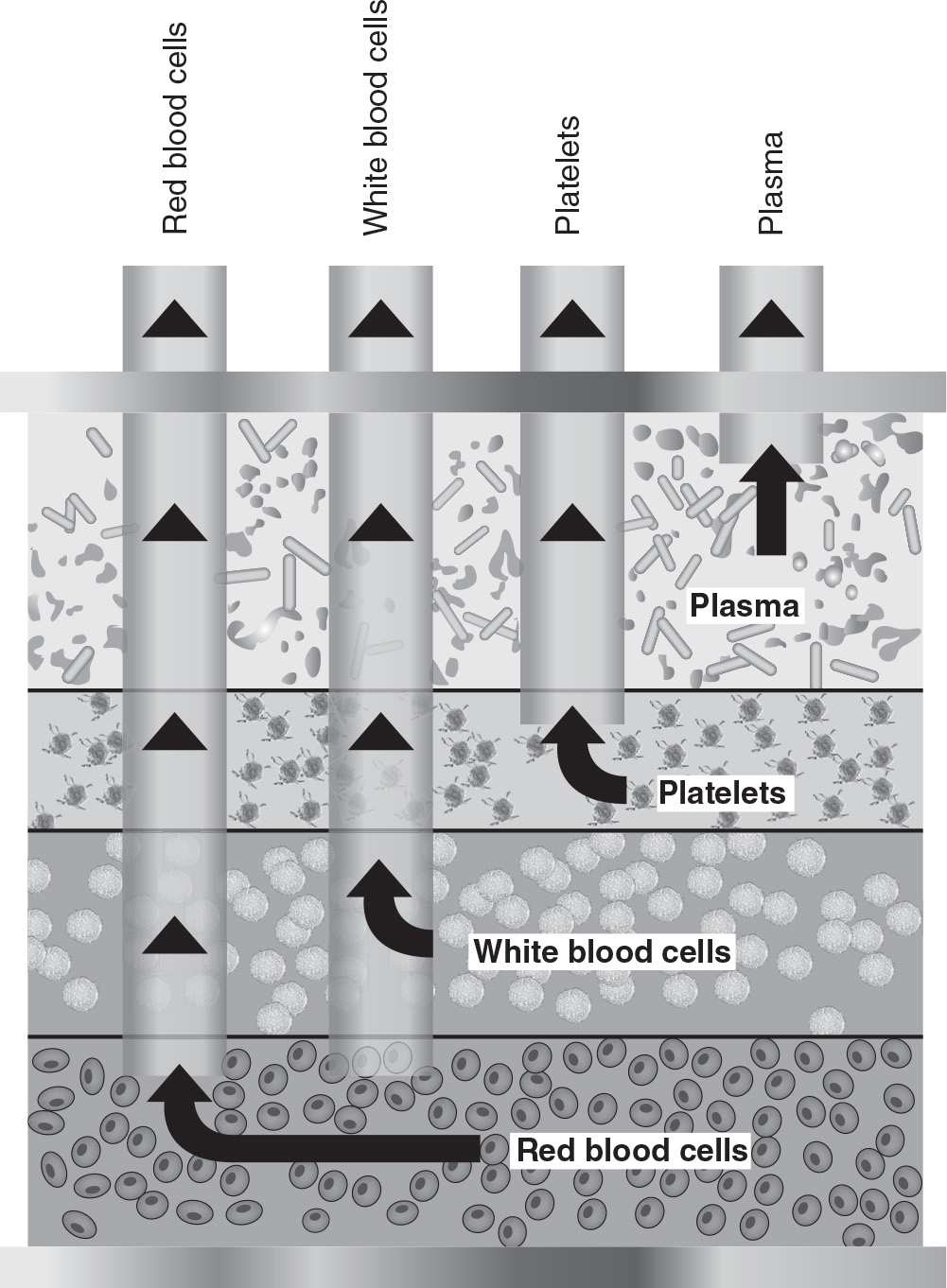
FIGURE 18.1 During centrifugal apheresis, plasma and cells are separated in layers depending on specific gravity. Each layer can be removed, depending on the procedure and fluid and/or cell replacement infused simultaneously. (Courtesy of Dobri Kiprov, MD. Reprinted from Linz W, et al. Principles of Apheresis Technology. 5th ed. American Society for Apheresis; 2014. www.apheresis.org.)
B. Membrane plasma separation (MPS). Membrane plasma separators are derived from the technology used in dialysis. Hollow-fiber filters for MPS look very similar to dialysis filters. It is easy to assume that one can simply exchange the dialysis filter with an MPS filter and perform a hemofiltration procedure without dialysate. However, removing plasma is physiologically different from removing ultrafiltrate. When water is removed from the intravascular compartment, extravascular fluid can diffuse in to buffer the volume removal. When plasma is removed from the intravascular compartment, refilling rate of the vascular compartment is reduced. Therefore, there is a higher risk of cardiovascular complications during plasma exchange. Equipment specifically designed for membrane plasma separation must be used to assure patient safety. Membranes with a molecular-weight cutoff of about 3 million daltons are used, which is sufficient to allow passage of immune complexes (MW ≈1 million). MPS filters can be manufactured in either a hollow-fiber or a parallel-plate configuration. An example of a hollow-fiber plasma separator is the Plasma-Flo made by Asahi (Apheresis Technologies, Palm Harbor, FL). The membrane allows plasma only to pass, as the pores are small enough to hold back the formed elements of the blood. The membrane has a sieving coefficient (ratio of concentration in filtrate to blood) between 0.8 and 0.9 for albumin, IgG, IgA, IgM, C3, C4, fibrinogen, cholesterol, and triglycerides (at a blood flow rate of 100 mL/min and a transmembrane pressure [TMP] of 40 mm Hg) (Fig. 18.2). A number of manufacturers offer either modified CRRT equipment or dedicated instruments for membrane plasmapheresis.
Membrane plasma separation (MPS) must be performed at low TMP (<500 mm Hg) to avoid hemolysis. With hollow-fiber devices, the blood flow rate should exceed 50 mL/min to avoid clotting. The ideal blood flow rate (Qb) is usually 100–150 mL/min. When the blood flow rate is 100 mL/min, a plasma removal rate of 30–50 mL/min can be expected. Thus, the average time required to perform a typical membrane filtration (Ve = 2,800 mL) is <2 hours (40 mL/min × 60 minutes = 2,400 mL/hr).
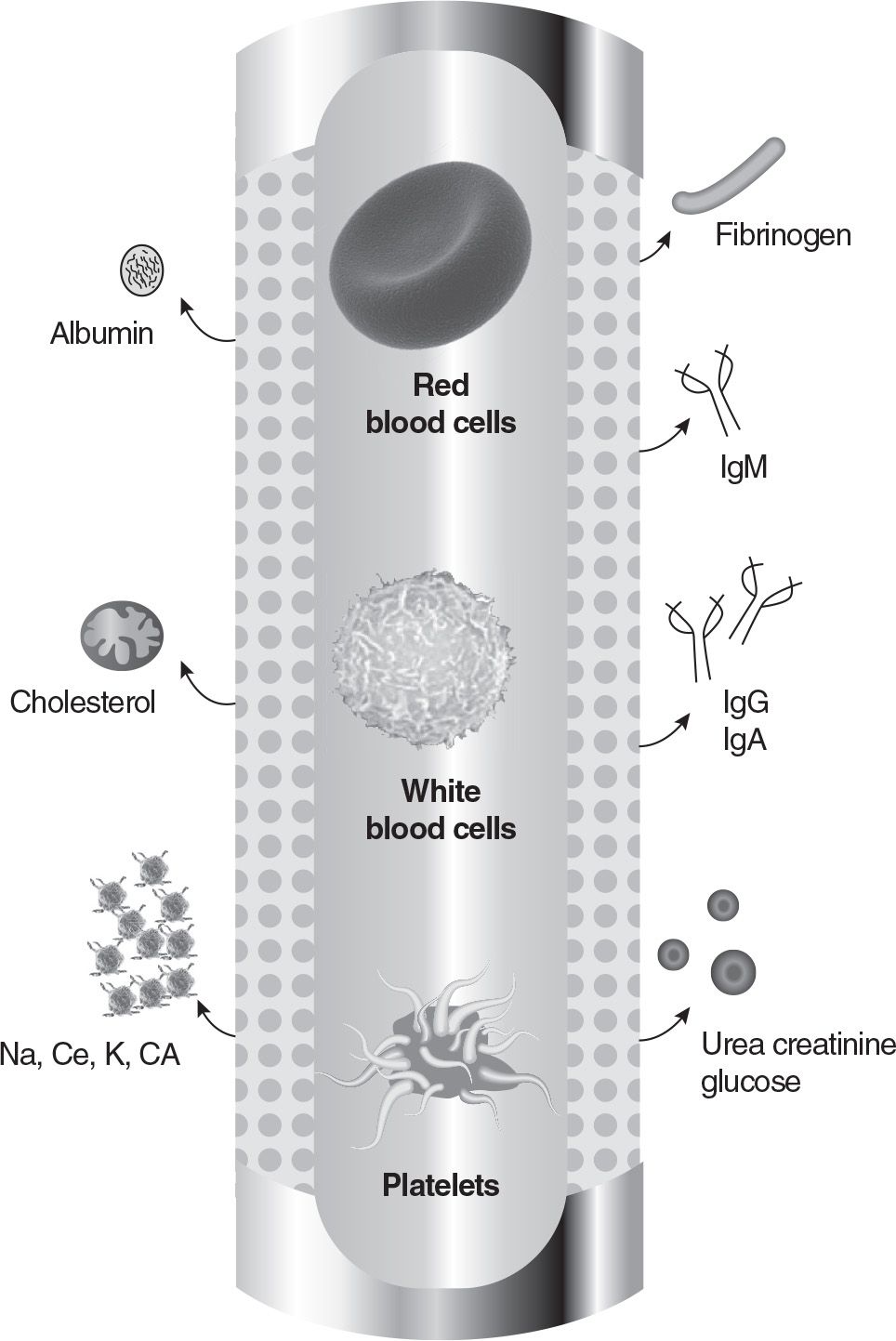
FIGURE 18.2 During membrane plasma separation, blood cells are not allowed to pass through the pores of the filter, while plasma constituents pass through. (Courtesy of Dobri Kiprov, MD. Reprinted from Linz W, et al. Principles of Apheresis Technology. 5th ed. American Society for Apheresis; 2014. www.apheresis.org.)
C. Comparison of membrane and centrifugation devices (Table 18.4). Centrifugal blood cell separators are the preferred therapeutic apheresis devices in the United States. These are capable of performing cytapheresis (leukapheresis, erythrocytapheresis, and thrombocytapheresis) in addition to plasmapheresis. Centrifugal devices also operate at lower whole-blood and plasma flow rates (Qb in the range of 40–50 mL/min). Such blood flows can be obtained from a large peripheral vein (antecubital vein), eliminating the risks associated with central vascular access in many cases.
MPS is faster for performing plasmapheresis. However, it is unsuitable for treating patients with the hyperviscosity syndrome due to paraproteinemia (most commonly Waldenström macroglobulinemia) or patients with cryoglobulinemia, because the available devices are not efficient in removing very large macromolecules. MPS normally is performed using heparin as an anticoagulant; when treating bleeding disorders such as TTP, heparin should not be used, and a citrate-based method is indicated instead.
IV. VASCULAR ACCESS. As noted earlier, for the centrifugal device systems, a Qb in the range of 40–50 mL/min is required. This can sometimes be obtained from a large peripheral vein (antecubital vein). On the contrary, a central venous access is needed when using MPS because a blood flow rate between 100 and 150 mL/min is required for the successful and efficient operation of the filtration system. For MPS the best approach is the use of a large-bore, dual-lumen catheter, similar to the ones used for dialysis and especially dedicated for apheresis. The majority of intravascular devices available for nondialysis use, such as Swan–Ganz catheters and triple-lumen catheters, almost never provide adequate blood flow for plasmapheresis, although they may be suitable for blood return.
Citrate infusion (see later) causes an acute reduction in the plasma ionized calcium level (in the face of normal total serum calcium level), which can have a local effect on the cardiac conduction system and can generate life-threatening arrhythmia, particularly when blood is returned centrally close to the atrioventricular node of the heart. Cardiac rhythm should be monitored, and blood-warming devices should be used, especially if processed blood is returned centrally.
When the nature of the disease requires chronic TPE (e.g., hypercholesterolemia, cryoglobulinemia), the creation of a permanent access is preferred. Patients may undergo placement of a central catheter for long-term use, or long-term access may be achieved using an arteriovenous fistula or polytetrafluoroethylene graft.
V. ANTICOAGULATION. Anticoagulation is mandatory for therapeutic apheresis procedures, whether by MPS or centrifugal devices. In general, filtration devices use heparin, whereas centrifugal machines require the use of citrate.
A. Heparin. Heparin sensitivity and half-life vary greatly in patients, and individual adjustment of dosage is necessary. Heparin doses may need to be increased in patients with low Hct (increased volume of distribution) and when the plasma filtration rate is high (a high plasma filtration rate results in increased net removal of heparin, which has a sieving coefficient of 1.0).
B. Citrate. Anticoagulant citrate dextrose (ACD) is used as the anticoagulation solution for most TPE procedures. Citrate chelates calcium, which is a necessary cofactor in the coagulation cascade, and this inhibits thrombus formation and platelet aggregation. ACD comes in two standard formulations. Formula A (ACD-A) contains 2.2 g/dL of sodium citrate and 0.73 g/dL of citric acid. Formula B (ACD-B) contains 1.32 g/dL of sodium citrate and 0.44 g/dL of citric acid. ACD-A is used for all continuous-flow centrifugal devices.
Although bleeding is uncommon with citrate, low plasma ionized calcium levels commonly occur. Therefore, patients must be carefully observed for symptoms and signs of hypocalcemia (perioral and/or acral paresthesias; some patients may experience shivering, light-headedness, twitching, tremors, and, rarely, continuous muscular contractions that result in involuntary carpopedal spasm). If plasma ionized calcium levels fall more severely, symptoms can progress to frank tetany with spasm in other muscle groups, including life-threatening laryngospasm. Grand mal seizures have been reported. These symptoms and signs may be accentuated by alkalosis due to hyperventilation. Reductions of ionized calcium values also lengthen the plateau phase of myocardial depolarization, manifested electrocardiographically by prolongation of the QT interval. Very high citrate levels, with corresponding low ionized calcium, lead to depressed myocardial contractility, which, though very rare, can provoke fatal arrhythmias in patients undergoing apheresis.
1. Prevention of low ionized calcium levels during citrate anticoagulation. The following measures can be considered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





