The Transplant Operation and Its Surgical Complications
Jeffrey L. Veale
Jennifer S. Singer
H. Albin Gritsch
Kidney transplantation is an “elective” surgical procedure performed in patients who have undergone careful preoperative assessment and preparation. Chronic dialysis enables patients to be maintained in optimal condition and provides time to address potentially complicating medical and surgical issues. In this respect, kidney transplantation differs from heart, lung, or liver transplantation, in which the condition of the patient is often deteriorating rapidly in the pretransplantation period.
TRANSPLANTATION OPERATION
Immediate Preoperative Preparations
If transplant candidates have been well prepared (see chapter 7), it is rarely necessary to call off surgery because of last-minute findings. Occasionally, cancellation of surgery is required because of recent events, such as new onset of chest pain or cardiographic changes, diabetic foot ulcers, peritonitis, pneumonia, or gastrointestinal bleeding.
The decision to dialyze a patient before transplantation depends on the timing of the previous dialysis, clinical assessment of volume status, and serum electrolyte levels, particularly potassium. Pretransplantation dialysis is associated with an increased incidence of delayed graft function. Because of the danger of intraoperative or postoperative hyperkalemia in oliguric patients, it is wise to dialyze patients with a serum potassium level of more than 5.5 mEq/L. In welldialyzed patients, preoperative dialysis for fluid removal is usually unnecessary. If fluid is removed, it should be done with care to maintain the patient at or somewhat above dry weight to facilitate postoperative diuresis. If time constraints demand it, a brief preoperative dialysis lasting 1 to 2 hours may be all that is necessary to reduce potassium levels and to optimize the hemodynamic status.
Operative Technique
Because all kidney transplant recipients receive immunosuppressive drugs, and because many are anemic or malnourished at the time of surgery, wound healing is potentially compromised. Meticulous surgical technique, attention to detail, strict aseptic technique, and perfect hemostasis are essential. Drains should be closed systems and should be removed as quickly as possible.
Incision
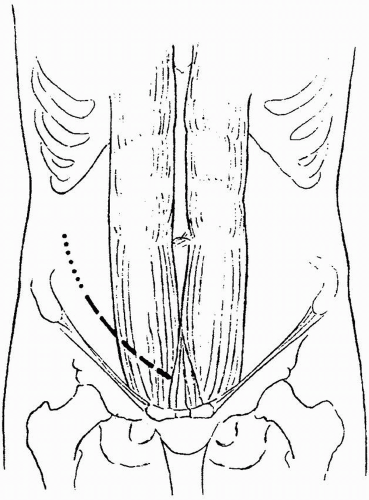
After the administration of prophylactic antibiotics, a lower abdominal Gibson incision is made (Fig. 8.1). It can be extended into the flank, or as high as the tip of the 12th rib, if more exposure is needed. In a first transplantation, the incision site may be in either lower quadrant. There are different approaches to the decision regarding which side to use. One approach is to always use the right side, regardless of the side of origin of the donor kidney, because the accessibility of the iliac vein makes the operation easier than on
the left side. Another approach is to use the side contralateral to the side of the donor kidney; that is, a right kidney is put on the left side, and vice versa. This technique was used when the hypogastric artery was routinely used for the anastomosis because the vessels lie in a convenient position and the renal pelvis is always anterior, making it accessible if ureteral repair is needed. The third approach is to use the side ipsilateral to the donor kidney; that is, a right kidney is put on the right side, and vice versa. This choice is best when the external iliac artery is used for the arterial anastomosis. The vessels then lie without kinking when the kidney is placed in position. In repeat transplantations, the side opposite the original transplant is generally used. In further transplants, the decision regarding where to place the kidney is more complex; a transabdominal incision may be necessary, and more proximal vessels may be used. In patients with type 1 diabetes who may be eventual candidates for pancreas transplantation, the kidney is preferentially placed in the left iliac fossa to facilitate a possible pancreas transplantation on the right side (see Chapter 15).
the left side. Another approach is to use the side contralateral to the side of the donor kidney; that is, a right kidney is put on the left side, and vice versa. This technique was used when the hypogastric artery was routinely used for the anastomosis because the vessels lie in a convenient position and the renal pelvis is always anterior, making it accessible if ureteral repair is needed. The third approach is to use the side ipsilateral to the donor kidney; that is, a right kidney is put on the right side, and vice versa. This choice is best when the external iliac artery is used for the arterial anastomosis. The vessels then lie without kinking when the kidney is placed in position. In repeat transplantations, the side opposite the original transplant is generally used. In further transplants, the decision regarding where to place the kidney is more complex; a transabdominal incision may be necessary, and more proximal vessels may be used. In patients with type 1 diabetes who may be eventual candidates for pancreas transplantation, the kidney is preferentially placed in the left iliac fossa to facilitate a possible pancreas transplantation on the right side (see Chapter 15).
Venous Anastomosis
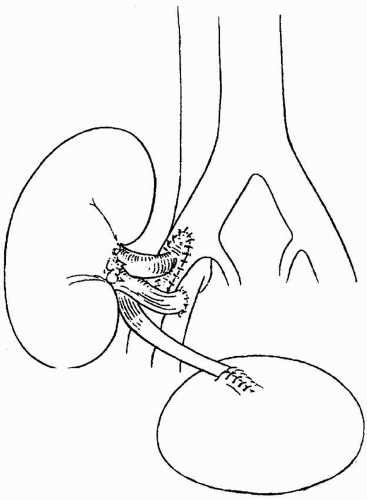
Using a 5-0 polypropylene suture, the donor renal vein is usually anastomosed end to side to the external iliac vein (Fig. 8.2). If there are multiple renal veins,
the largest may be used; the others can be ligated safely because of internal collateralization of the renal venous drainage. If two veins are about the same size, they can be sewn together with the “pair of pants” technique, or individually anastomosed to the external iliac vein. With deceased donor renal transplants, the donor vena cava may be used as an extension graft for the short, right renal vein. The venous anastomosis is usually done first to minimize ischemia to the leg.
the largest may be used; the others can be ligated safely because of internal collateralization of the renal venous drainage. If two veins are about the same size, they can be sewn together with the “pair of pants” technique, or individually anastomosed to the external iliac vein. With deceased donor renal transplants, the donor vena cava may be used as an extension graft for the short, right renal vein. The venous anastomosis is usually done first to minimize ischemia to the leg.
Arterial Anastomosis
The donor renal artery is usually sewn to the external iliac artery in an end-toside fashion using a 5-0 or 6-0 polypropylene suture (Fig. 8.2). In a deceased donor kidney transplantation, the donor renal artery or arteries are usually kept in continuity with a patch of donor aorta called a Carrel aortic patch,
which makes the end-to-side anastomosis much easier and safer, and facilitates the anastomosis of multiple renal arteries. In a living related donor transplantation, a Carrel patch is not available, and the renal artery is sewn to the recipient artery. In small children and in patients undergoing repeat transplantation on the same side, it may be necessary to use arteries other than the external iliac artery. The aorta, common iliac artery, or hypogastric artery is sometimes used. During the anastomosis time, the kidney is wrapped in a gauze pad with crushed ice saline to minimize warm ischemia.
which makes the end-to-side anastomosis much easier and safer, and facilitates the anastomosis of multiple renal arteries. In a living related donor transplantation, a Carrel patch is not available, and the renal artery is sewn to the recipient artery. In small children and in patients undergoing repeat transplantation on the same side, it may be necessary to use arteries other than the external iliac artery. The aorta, common iliac artery, or hypogastric artery is sometimes used. During the anastomosis time, the kidney is wrapped in a gauze pad with crushed ice saline to minimize warm ischemia.
Multiple Arteries
A variety of techniques have been proposed for handling donors with multiple renal arteries. In no case should a lower pole artery be sacrificed because this may lead to ureteral necrosis. There may be visible capsular vessels that supply a tiny part of the cortical surface of the kidney. These vessels may be ligated, and tiny superficial ischemic areas on the surface of the kidney may result. In deceased donor transplantations, it is best to keep all the arteries on a single large Carrel aortic patch and to sew the Carrel patch to the recipient vessel. If there are multiple arteries in a living donor transplant, or if a Carrel patch is not available, the donor arteries can be anastomosed individually or anastomosed to each other before being anastomosed to the recipient vessel. Occasionally, a small lower-pole branch may be anastomosed end to end to the inferior epigastric artery. For recipients with multiple donor arteries, it may be helpful to administer 1000 units of intravenous heparin by bolus before suturing the arterial anastomoses, with a continuation of the heparin infusion at 100 units per hour for the duration of the hospitalization.
Ureter Anastomosis
The ureter can be anastomosed to the recipient bladder or into the ipsilateral native ureter as a ureterostomy. The native ureter may also be brought up to the allograft renal pelvis as a ureteropyelostomy. Most surgeons use the bladder whenever possible. Preferably, the recipient’s bladder will have been shown to be functional before the transplantation; however, even small, contracted bladders that have not “seen” urine for prolonged periods usually regain function and capacity. If necessary, the ureter can be connected to a previously fashioned ileal or colonic conduit.
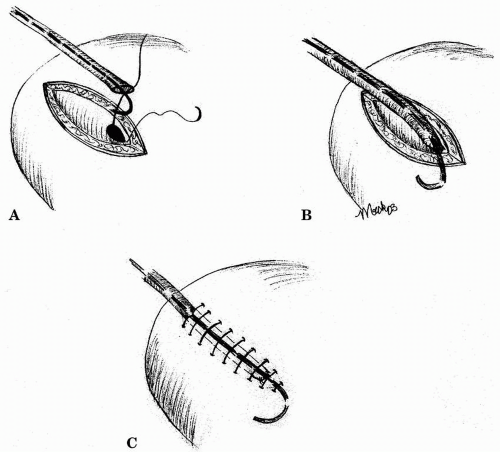
There are several ways of reimplanting the ureter into the bladder. The most common approach is one in which the ureter is reimplanted extravesically, using the Lich-Gregoir technique. First, the bladder is distended with saline, and the extravesical tissues are dissected from the detrusor muscle. A muscular tunnel is then created by separating the detrusor muscle from the bladder mucosa for a length of about 2 to 4 cm. The ureter is prepared by removing redundant ureteral length, preserving adequate distal blood supply, and spatulating posteriorly. A mucosal opening is created, and interrupted or running degradable suture, preferentially polydioxanone surgical suture, is used to approximate the ureteral and bladder mucosa. Finally, the detrusor muscle is closed exteriorly to create an antireflux mechanism (Fig. 8.3). Absorbable suture is used to prevent stone formation. Foley catheter drainage of the bladder is required for about 4 days, unless there are bladder abnormalities that may necessitate longer drainage.
Other, less common approaches include the Barry technique, the singlestitch Taguchi technique, and the intravesical Leadbetter-Politano technique. Whichever technique is used for the ureteral anastomosis, an indwelling stent should be placed in most cases. In a recent meta-analysis by Mangus and Haag, the urinary complication rate following routine stenting was 1.5% compared with 9% without stenting (P < .0001). Routine stenting has also proved
cost-effective because of the hospital costs associated with a single urinary complication. Additionally, stents are well tolerated by transplant recipients because the location of the ureter high in the bladder dome and kidney denervation result in minimal trigonal irritation and reflux pain. With the use of cotrimoxazole for prophylaxis against Pneumocystis carinii, and the removal of stents 3 to 4 weeks after transplantation, the incidence of urinary tract infections among stented recipients remains low. Clear notation of stent placement and its subsequent removal must be made to prevent inadvertent stent retention. Because a retained stent may be difficult to remove intact and may be a source of recurrent urinary tract infections and ureteral stones.
cost-effective because of the hospital costs associated with a single urinary complication. Additionally, stents are well tolerated by transplant recipients because the location of the ureter high in the bladder dome and kidney denervation result in minimal trigonal irritation and reflux pain. With the use of cotrimoxazole for prophylaxis against Pneumocystis carinii, and the removal of stents 3 to 4 weeks after transplantation, the incidence of urinary tract infections among stented recipients remains low. Clear notation of stent placement and its subsequent removal must be made to prevent inadvertent stent retention. Because a retained stent may be difficult to remove intact and may be a source of recurrent urinary tract infections and ureteral stones.
Drains
Drains may be placed through a separate small incision into the perirenal space to drain blood, urine, or lymph. Some surgeons routinely place drains, whereas others do not. Closed drains, such as the Jackson-Pratt type, are preferred over the open Penrose-type drains because of a lower risk for wound infection. Placing a drain at the end of the procedure and leaving it for the initial postoperative course has been shown to reduce the incidence of lymphoceles. Drains should typically be removed once the output is less than 100 mL per day.
Intraoperative Fluid Management
Adequate perfusion of the newly transplanted kidney is critical for the establishment of an immediate postoperative diuresis and the avoidance of delayed
graft function (see Chapter 9). Volume contraction should be avoided and mild volume expansion maintained, conducive to the recipient’s cardiac status. Central venous pressure should be maintained at about 12 mm Hg with the use of isotonic saline and albumin infusions, and mean arterial pressure should be kept above 80 mm Hg.
graft function (see Chapter 9). Volume contraction should be avoided and mild volume expansion maintained, conducive to the recipient’s cardiac status. Central venous pressure should be maintained at about 12 mm Hg with the use of isotonic saline and albumin infusions, and mean arterial pressure should be kept above 80 mm Hg.
Before the release of the vascular clamps, a large dose of methylprednisolone is usually given. If an antibody induction agent is being used (see Chapter 5), it should be administered before this time. Mannitol and furosemide are also given, and fluid replacement is maintained accordingly. Postoperative management is discussed in Chapter 9.
En Bloc and Dual Kidney Transplantation
At the extremes of donor age, both donor kidneys are sometimes transplanted into a single recipient. The simultaneous use of both kidneys entails some additional technical risks to the recipient. Their use is a reflection of the donor shortage and reluctance to discard functional organs.
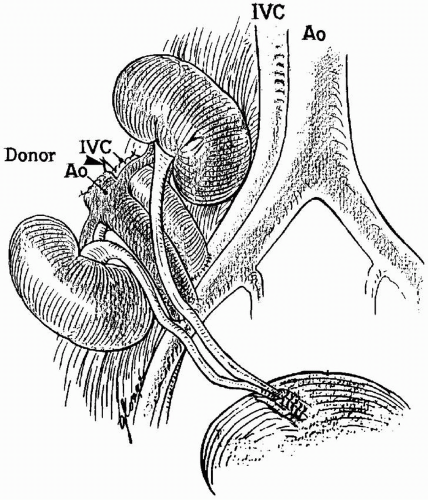
For donors younger than 2 years of age, both kidneys are usually transplanted en bloc with the donor aorta and vena cava (Fig. 8.4). For donors between the ages of 2 and 5 years, the surgeon decides whether there is sufficient nephron mass to separate the kidneys and provide allografts for two individuals. Separation can be considered when the allograft measures greater than
6 cm in length (the donor weight is usually > 15 kg). For the en bloc procedure, the aorta and vena cava superior to the renal vessels are typically closed with 6-0 nonabsorbable monofilament suture. All the other branches of the great vessels are carefully ligated with 4-0 silk ties, the infrarenal aorta is then anastomosed to the external iliac artery, and the infrarenal vena cava is anastomosed to the external iliac vein. The renal vessels should not be compromised when oversewing the suprarenal aorta and vena cava.
6 cm in length (the donor weight is usually > 15 kg). For the en bloc procedure, the aorta and vena cava superior to the renal vessels are typically closed with 6-0 nonabsorbable monofilament suture. All the other branches of the great vessels are carefully ligated with 4-0 silk ties, the infrarenal aorta is then anastomosed to the external iliac artery, and the infrarenal vena cava is anastomosed to the external iliac vein. The renal vessels should not be compromised when oversewing the suprarenal aorta and vena cava.
The kidneys from pediatric en bloc donors must be carefully positioned to avoid kinking of the blood vessels, and the ureters should be greater than 6 cm to reach the bladder without tension. If the ureters are implanted into the bladder separately, and a complication occurs in one kidney, then the risk for compromising the other kidney is reduced. The rate of technical complications, most typically urine leaks and vascular thrombosis, varies between 10% and 20% with young donor kidneys transplanted individually or en bloc.




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree