Fig. 7.1
This MR image shows a T3N1V1 mid rectal tumour with a potentially involved circumferential margin. There is a plaque of high signal intensity in the presacral space. The patient subsequently underwent long course chemoradiotherapy
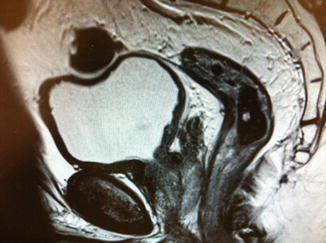
Fig. 7.2
This is an MR image post long course chemoradiotherapy. There has been a good response with the previously involved nodes no longer evident. The changes in the presacral region have disappeared. Histopathology of the subsequently resected specimen reported ypT1N0V0 R0 Mandard tumour regression grade 2
Tumor downstaging as a result of chemoradiation may be utilized in order to achieve sphincter preservation in those with low rectal cancer threatening the sphincter complex. However, current evidence for this specific role is not clear and the use of chemoradiation in order to achieve this goal remains controversial [22]. It is our practice, as we have already stated, to treat patients according to their original pre-treatment MRI images. We would therefore not use neoadjuvant treatment for the purpose improving our rate of sphincter preservation.
It is important to recognize that some rectal cancers behave biologically very differently to others. Clinicians treating rectal cancer should aim to identify those patients who respond to neoadjuvant treatment and perhaps more importantly the small proportion who will progress despite this therapy and require early surgery. Over recent years, there has been a focus on trying to identify prognostic molecular biomarkers in rectal cancer in an attempt to predict response to chemoradiation. It is hoped that in the future therapies can be tailored to the tumor biology of each individual patient [1]. However a present we do not have this luxury and must use existing clinical and radiological tools to define the extent of tumor response to neoadjuvant treatment.
Monitoring tumor response to neoadjuvant treatment can be challenging. Size and shape-based criteria can be lacking in accuracy when trying to discriminate between responders and non-responders [23, 24]. One of the most accurate tools for monitoring response is the MRI defined tumor regression grade, which appears to be able to predict long-term outcomes in terms of local recurrence and 5-year survival [25]. Sequential imaging with this modality has the advantage of being able to quantify response to chemoradiation and may be used to predict the appropriate timing of surgery based on level of response.
Interval Between Completion of Neo-adjuvant Treatment and Surgery
The timing of surgery post neo-adjuvant treatment remains an area for further research effort. Currently the only randomized trial to tackle this question is the Lyon R90-01 trial published in 1999 [26]. This study included over 200 patients with rectal cancer who were randomized to surgery either within 2 weeks of completing their radiotherapy or surgery between 6 and 8 weeks of completing treatment. The group who underwent surgery following a longer interval (6–8 weeks) had significantly more clinical tumor response and tumor downstaging when compared with those who received surgery within 2 weeks of radiotherapy. These findings have influenced standard US and UK practice and until recently it has remained routine to wait between 6 and 8 weeks post neo-adjuvant treatment before proceeding with surgery. More recently however this standard interval has been challenged as it appears that waiting for longer than 8 weeks may allow a higher degree of tumor necrosis and regression.
Surgeons from the Cleveland Clinic have studied a cohort of over 240 patients and identified a significantly better pathological complete response (pCR) rate in those waiting over 8 weeks between completing neo-adjuvant treatment and undergoing surgery [27]. Multivariate analysis revealed time-interval between completion of treatment and surgery to be the only predictor of pCR. A follow-up study determined that waiting for over 8-weeks was safe and was not associated with higher peri-operative morbidity or mortality. This longer time-interval was associated with a lower 3-year local recurrence rate [28].
A study from Nottingham in the UK looked at tumor regression related to neo-adjuvant treatment and calculated the tumor-halving time for rectal cancer to be 14 days [29]. These findings were based on the tumor volume difference between pre-treatment CT imaging and post-operative histopathology measurements. It was estimated that from beginning neoadjuvant treatment it would take an average sized tumor 20-weeks to regress fully, based on these findings. One must remain aware however that each individual patient will respond differently to chemoradiation. Some may respond far quicker whilst others will fail to respond at all and may even progress despite neoadjuvant therapy.
There is a prospective trial that is currently recruiting and is being run by the Royal Marsden NHS Foundation Trust in London. The primary aim of this study is to identify whether waiting 12 weeks from completion of chemoradiotherapy results in greater tumor downstaging or tumor regression when compared with an interval of 6 weeks. Secondary outcome measures will include the proportion of patients undergoing sphincter-saving surgery and the peri-operative morbidity and mortality rates. There is also another prospective study called “A trial looking at surgery following treatment for rectal cancer (STARRCAT)” which is also recruiting and is also comparing intervals of 6 and 12 weeks. The aims of this study however are to assess surgical difficulty and complexity when surgery is delayed and also to evaluate patient experience and the side-effects of treatment. The results of these studies may help to ascertain the optimum time-interval between completion of chemo-radiotherapy and surgery.
Clinical and Pathological Complete Response
Significant downstaging of rectal cancers will occur in a substantial proportion of patients treated with neo-adjuvant chemoradiation, and in some cases the tumor will be entirely sterilized. Some studies have reported that up to 25 % of patients will have a pathological complete response (pCR) following this form of treatment [19–21]. pCR is defined as the complete absence of adenocarcinoma cells within the surgical specimen when examined by a histopathologist (i.e. stage: ypT0 N0).
A pooled analysis of individual patient data from 27 existing articles suggested that those patients who achieve a pCR had significantly better 5-year disease free survival rates when compared with those who failed to achieve such a good response [30]. A systematic review and meta-analysis of existing evidence including a total of 3,363 patients with either stage II or stage III rectal cancer and with a mean follow up of 55.5 months identified significantly better outcomes in patients who achieved a pCR when compared with those who only achieved an incomplete response [31]. Those with a pCR where approximately four times less likely to develop local recurrence and also over four times less likely to develop distant disease. They were more than four-times more likely to be disease free at 5 years and had a 3.3 fold overall survival advantage when compared with incomplete or non-responders. The findings of this meta-analysis suggest that following pCR the risk of local recurrence at a mean follow-up of 55.5 months is 0.7 %. If this is the case, then pCR following neoadjuvant chemoradiation virtually eradicates the risk of local recurrence. pCR was shown to be associated with an overall 5-year survival rate of 90.2 % and a disease free survival rate of 87 %. These results are comparable to those following an R0 resection for stage I rectal cancer [31]. One should be aware however, that the majority of the studies included in this analysis are retrospective case-series and that there is currently no level 1 evidence to support these findings. Despite this it seems logical to expect patients who respond well to chemoradiation and then undergo surgery to remove the rectum to do better than patients who fail to respond so well to neoadjuvant treatment.
There are many different approaches to the management of patients who achieve a pCR post neoadjuvant treatment throughout the world. There are some who would recommend less radical surgery for selected patients with pCR, thus avoiding the need for an anterior resection or AP resection of the rectum. There are reported series of transanal excision and the use of transanal endoscopic microsurgery (TEMS) to excise the scars left behind post neoadjuvant treatment in patients who appear to have achieved a clinical complete response (cCR) to treatment [32–35]. Unfortunately, as with much of the data relating to patients with a pCR, many of these reports are from small case-series and much of the data has been gathered retrospectively. There is currently no high level evidence to support this practice.
There are also advocates of an expectant (“watch and wait” or “wait and see”) approach to the management of patients who achieve a cCR post neoadjuvant treatment. In particular, Habr-Gama and her colleagues from Sao Paulo in Brazil have published widely with regards to this approach [36–41]. Their approach includes intensive clinical, radiological and endoscopic follow-up post neoadjuvant treatment. In those patients deemed to have achieved a cCR, defined as the absence of clinically detectable residual tumor, an expectant (non-operative) approach is adopted. Conversely, those who are assessed and have failed to achieve a cCR are recommended to undergo rectal resection.
The appeal of an expectant approach to the management of patients with rectal cancer who undergo a cCR following neo-adjuvant therapy is understandable. Those in question are usually patients with low rectal cancer who would normally require significant pelvic surgery in the form of a low anterior resection or AP excision. Surgery of this type carries with it a risk of morbidity and mortality, with potential long-term side effects in terms of bowel, urinary and sexual dysfunction and a significant change of a temporary or permanent stoma. Avoiding these potential hazards can be understandably appealing to patients and their surgeons. However the longer-term uncertainties associated with the “watch and wait” approach must also be considered.
There are a number of unanswered questions associated with the approach of Habr-Gama and her colleagues, reflected in the fact that this strategy has not been adopted more widely in the field of colorectal surgery. One needs to clarify what constitutes a cCR and how accurately does this predict a pCR. Habr-Gama and her colleagues recognize the difficulty related to defining what constitutes a cCR and the imprecision and variation of this definition between different authors [42]. Currently, there is no standardized definition of what constitutes a cCR.
In a paper from 2010, Habr-Gama and colleagues have listed a number of observed clinical and endoscopic findings in patients who frequently have a cCR [42]. Subtle features such as whitening of the mucosa, telangiectasia at the site of the tumor and a loss of pliability of the rectal wall harboring the scar are thought to predict a cCR. Conversely, ulceration, a palpable nodule or stenosis at the site of the previous tumor are thought to predict an incomplete clinical response and the need for definitive surgery. Biopsies are thought by Habr-Gama to be of limited clinical value [43]. Whereas positron emission tomography/computed tomography (PET/CT) performed at 12 weeks post neoadjuvant treatment is considered a useful modality in the assessment and diagnosis of residual disease [44].
In a Dutch series where a “watch and wait” approach was adopted, cCR was defined according to a number of strict criteria. These included the clinical absence of palpable or visible disease, the absence of suspicious lymph nodes at MRI, no disease or a small scar or ulcer at endoscopy and negative biopsies from the scar. Only if all of these criteria were met, was the patient considered to have achieved a cCR [45]. Currently, it seems that there is no widespread consensus amongst colorectal surgeons as to the definition of a cCR. Indeed when members of the Association of Coloproctology of Great Britain and Ireland were sent a questionnaire on the subject, they replied with over 70 different combinations of investigations and imaging modalities to define a cCR [46]. At present there is a need for greater clarity and standardization of the definition of a cCR, before more widespread adoption of this management strategy can be recommended.
There is also a potential for patients with an apparent cCR to harbor disease within their lymph nodes. Up to 17 % of patients will have no intraluminal evidence of residual disease and at pathology no mural evidence of cancer (ypT0) but will harbor cancer cells within the lymph nodes [47]. Conversely, there will be some patients (8.3 % according to Habr-Gama et al. [37]) who clinically appear to have evidence of residual disease who in fact pathologically will have achieved a pCR. Clinically, endoscopically and radiologically predicting pCR remains challenging at best and even in the hands of very experienced surgeons with patients undergoing intensive follow-up it remains fraught with difficulty. Future advances in radiology, biochemistry and molecular biology may enable more accurate prediction of pCR in those with a cCR and may eventually obviate the need for radical surgery and its potential morbidity in this group of patients [31].
At present, the “watch and wait” strategy remains experimental. In addition to the points already discussed, there are concerns regarding limitations of many of the reporting studies. The majority of these studies are small retrospective series with insufficiently long and rigorous follow-up. There have been concerns raised regarding the fact that up to 20 % of patients with an apparent cCR will fail non-operative treatment within the first year and will require salvage surgery [1]. There is a lack of data specifically relating to these failures, their management and their eventual outcome. There is also a lack of data relating to quality of life and functional outcomes of patients undergoing non-operative treatment post neo-adjuvant treatment. Well-designed, prospective observational studies have been recommended to answer some of the questions regarding this expectant management approach [48].
Well-designed, prospective trials attempting to resolve some of these unanswered questions are already in progress. There is a study sponsored by the Royal Marsden NHS Foundation Trust (NCT01047969) that is recruiting patients currently and is aiming to assess the safety of omission of surgery following neo-adjuvant treatment. The primary outcome measures are to estimate the percentage of patients who can safely omit surgery, (defined as the percentage of patients at 2 years after the end of chemoradiation who have not had surgery and who are in cCR) and to prove the safety of deferred surgery, (as measured by the percentage of patients who have local failure at 2 years), where local failure is defined as positive margin status of resected tumor or surgically unsalvageable disease. Unfortunately, definitive results from this study are unlikely to be available before 2019. A Danish study is also currently recruiting patients in order to answer similar questions regarding the policy of “watchful waiting” (NCT00952926). This prospective study aims to calculate the frequency of local recurrence, the frequency of distant metastases and the overall 5-year survival in patients treated non-operatively following a cCR.
We would recommend awaiting the findings of these prospective trials before adopting a “watch and wait” approach in those with a cCR. This does not mean that a non-operative approach following neo-adjuvant therapy can never be adopted. There may be the exceptional case where an expectant management approach is preferable. For instance in a frail, unfit patient who has achieved a cCR and in whom the risks of surgery outweigh the potential benefits. In this type of case, a non-operative strategy may be discussed at MDT and with the patient and their family. However in general, and in view of the current level of available evidence the widespread adoption of a “watch and wait” policy in those achieving a cCR cannot be justified.
Side Effects and Surgical Implications of Neoadjuvant Chemoradiation
The reduced risk of local recurrence associated with the use of neoadjuvant chemoradiation is offset somewhat by its potential short-term and long-term complications. From a surgeons perspective, one will be familiar with the intra-operative effects of radiotherapy on pelvic tissues. This treatment can affect the pliability of tissues and make dissection along recognized tissue planes more challenging. There is also a tendency for greater intra-operative haemorrhage in those who have received neoadjuvant treatment [49]. There is also thought to be a higher risk of anastomotic leakage following neoadjuvant chemoradiation, which should be remembered when considering decisions regarding restorative surgery and in decisions regarding the use of a defunctioning ileostomy [49, 50].
The early post-operative complications of neo-adjuvant chemo-radiation include a higher rate of wound infection, wound dehiscence, anastomotic leakage, thrombosis and bowel obstruction [49]. Wound breakdown can be particularly problematic for those patients who have undergone an abdomino-perineal excision of the rectum post neo-adjuvant treatment. The perineal wound is prone to impaired healing in those who have received pelvic radiotherapy. The Medical Research Council CR07 trial which compared preoperative radiotherapy with selective postoperative chemo-radiotherapy identified a substantial increase in the rate of delayed perineal wound healing in those who had undergone an AP resection for rectal cancer following pre-operative radiotherapy (36 %) compared with those who received adjuvant treatment alone (22 %) [51]. Some wounds may have failed to heal up to a year or more post-surgery [52]. The potentially higher peri-operative risks associated with chemo-radiation should be considered by clinicians and explained to patients, in order for them to make an informed decision about whether to receive neo-adjuvant treatment or not.
Chemo-radiation is also associated with acute toxicity in a substantial proportion of patients. A Cochrane review comparing pre-operative chemoradiation versus radiation alone identified an incidence of grade 3 or grade 4 acute treatment related toxicity 14.9 % of patients treated with chemoradiation and a rate of 5.1 % in those treated with radiotherapy alone [53]. Grade 3 toxicity indicates that intervention other than medications is necessary to treat the side effect whereas grade 4 toxicity involves hospitalization for treatment of the problem. The EORTC study observed either grade 3 or grade 4 toxicity in 7.4 % of the patients treated with radiotherapy alone and in 13.9 % of patients who underwent neoadjuvant chemoradiation [54]. Similar findings were observed in a Polish trial comparing the effects of short course radiotherapy versus long-course chemoradiation with grade 3 or 4 toxicity occurring in 18.2 % of those receiving chemoradiation compared with 3.2 % of those receiving radiotherapy alone [55].
Acute toxicity is observed significantly more frequently in those receiving neo-adjuvant chemo-radiation when compared with those receiving similar doses of radiotherapy alone [54, 55]. Acute treatment-related toxicity may cause interruptions in neo-adjuvant therapy and in some patients may result in them failing to complete the course of therapy. This significant potential for toxicity associated with neo-adjuvant therapies must be considered by multi-disciplinary panels and should be explained and discussed thoroughly with patients. Accurate pre-treatment staging is essential in order to ensure that only appropriate patients are considered for this potentially morbid pre-operative therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






