Noncontrast scan first to assess for intramural hematoma
CTA (arterial phase) – 4 mL/s injection rate, bolus tracking at level of descending aorta + 15 s delay
No oral contrast
2 mm slice thickness, 1 mm reconstruction interval
Sagittal and coronal MPRs, 3D post-processing for endoluminal measurements
Delayed images when suspected alternative diagnosis, aortitis, or prior endoluminal graft repair
When endoluminal repair of an aortic aneurysm is being considered, it is important for the radiologist not only to report the maximum aortic diameter orthogonal to the long axis of the aorta but also to provide measurements of the aorta at the proximal and distal attachment sites, the length and morphology of the aneurysm neck, and the diameter of the iliac and femoral arteries, all of which affect the approach and potential for endovascular repair [2–4].
Many modern scanners automatically aid in selecting an appropriate kVp for the clinical application and patient size. When patient size permits, a lower kVp (100 or 80 when possible) should be considered for CT angiography to maximize the photoelectric effect of iodine and provide high-quality arterial opacification [5]. Dual-energy CT may be a useful adjunct in evaluating the abdomen as it permits the generation of virtual noncontrast images without the added time or radiation dose associated with conventional precontrast scanning [6]. When available, iterative reconstruction techniques can also be employed to reduce dose prospectively. However, radiation dose considerations such as these should not compromise image quality, as acute aortic conditions are potentially life threatening and more commonly seen in older patients for whom radiation dose is not a primary consideration.
Imaging Findings
Ruptured Abdominal Aortic Aneurysm
The primary imaging findings of AAA rupture often are readily apparent and include a retroperitoneal hematoma extending directly from the aneurysm and active extravasation of contrast material (Fig. 7.1) [7]. The vast majority of ruptured AAA patients present with hemorrhage that extends into the retroperitoneum, and intraperitoneal hemorrhage is far less common. Primary AAA rupture into the inferior vena cava as aortocaval fistula or gastrointestinal tract as aortoenteric fistula is also rare. Although contained AAA rupture can also present with the abovementioned findings, impending rupture may have much more subtle findings, including perianeurysmal soft tissue stranding. Additional CT findings associated with AAA rupture or impending rupture include the hyperattenuating crescent, draped aorta, and tangential calcium signs. Rapidly increasing aneurysm diameter and focal discontinuity of intimal calcification within the wall of the aneurysm also can be indications of aneurysm instability.
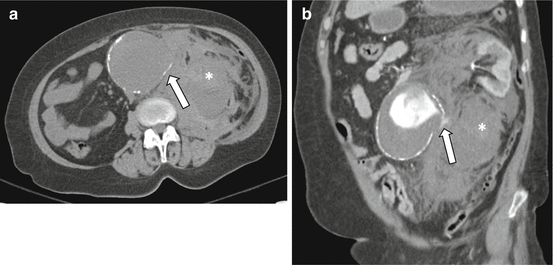

Fig. 7.1
Ruptured abdominal aortic aneurysm. Noncontrast transaxial (a) and contrast-enhanced coronal (b) CT images demonstrate the “tangential calcium” sign with focal disruption of intimal calcification which points at a tangent from the expected aortic circumference (a, arrow). Also note the contained active extravasation (b, arrow) and the large left-sided retroperitoneal hematoma (*)
A “hyperattenuating crescent” refers to a crescentic area of high attenuation within the wall or mural thrombus of an aneurysm secondary to penetration of blood from the aneurysm lumen into the aortic wall or mural thrombus (Fig. 7.2) [8]. By definition, a “hyperattenuating crescent” has a higher CT attenuation than the luminal blood on unenhanced scans and a higher attenuation than the skeletal muscle on postcontrast scans but is not as high as the attenuation of calcium [9]. Although the reported prevalence of this finding in patients with AAA rupture ranges from 21 to 77 %, the specificity has been reported to be as high as 93% [9, 10].
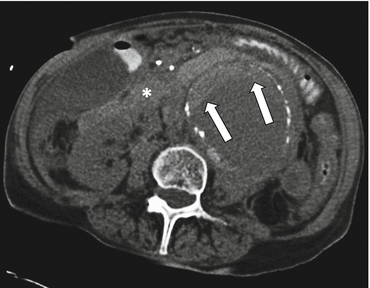

Fig. 7.2
Hyperattenuating crescent sign. Transaxial noncontrast CT image shows an infrarenal aortic aneurysm with a crescent-shaped area of high attenuation in the mural thrombus (arrows), representing acute hematoma. There is also rupture, evidenced by surrounding retroperitoneal hematoma (*)
A “draped aorta” describes the appearance of an AAA wall that bulges posteriorly, either unilaterally or bilaterally, and has lost its fat plane with the adjacent psoas muscle and vertebra (Fig. 7.3). This finding is a sign of aortic wall insufficiency and impending rupture, but also can be seen with a contained aortic leak [11, 12].
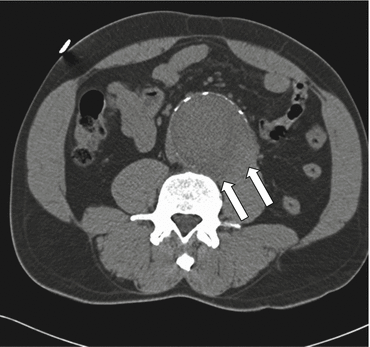

Fig. 7.3
Draped aorta sign. Precontrast transaxial CT shows abnormal contour of the aortic lumen and loss of the fat plane between the aorta and the left psoas muscle (arrows), the “draped aorta” sign in an aneurysm with contained rupture
Focal discontinuity of intimal calcification within an AAA wall, particularly when shown to be a new finding in comparison with prior studies, has been shown to be a useful sign of acute or impending rupture [9]. The “tangential calcium” sign, defined as calcified atherosclerotic plaque adjacent to an area of focal calcium discontinuity that is divergent from the normal arc of the aorta, is another finding highly associated with AAA rupture (Fig. 7.1) [13].
Intramural Hematoma
Aortic intramural hematoma (IMH) represents blood within the aortic wall without persistent flow or an intimomedial flap. Whether this blood arises due to rupture of the vasa vasorum or thrombosis of a dissection is somewhat controversial but probably irrelevant from the perspective of the radiologist as both potential etiologies result in the endpoint of IMH, a condition which is managed similarly to dissection [14, 15]. IMH usually resolves spontaneously, but it may progress to classic dissection, focal aortic ulceration, aortic aneurysm, aortic dissection, and rarely rupture [15, 16]. Factors that predict progression of an IMH to dissection or rupture include the presence of an aortic aneurysm, an ulcer-like projection, persistent symptoms after treatment, and progressive increase in aortic wall thickness and/or aneurysm diameter [17, 18]. IMH most commonly occurs in the descending thoracic aorta, rarely in the abdomen alone.
IMH is seen on imaging as an eccentric, crescent-shaped collection of blood in the aortic wall. On CT, this feature is best seen as hyperattenuation (40–70 HU) on precontrast images, particularly with the use of narrow window settings (Fig. 7.4) [19]. On MRI, precontrast T1-weighted or black blood images may be useful to demonstrate intramural blood products, which can be iso- or hyperintense to the skeletal muscle, depending on the age of the hematoma [20]. Interpretation based on viewing postconstrast images alone may be problematic as the intramural hematoma is less conspicuous when adjacent to the brightly enhancing aortic lumen, thus potentially being confused for mural thrombus. Imaging features that support mural thrombus rather than an IMH include an irregular intraluminal surface (IMH typically is smoothly contoured), isolation to a dilated portion of the aorta (IMH is more common in a normal diameter aorta), and the presence of multiple, interrupted lesions [15, 20]. Imaging features that help distinguish IMH from aortic dissection include the lack of enhancement of IMH, whereas delayed images generally depict some flow in the false lumen of a classic dissection. Fixed, medial displacement of intimal atherosclerotic calcification rather than a spiraled appearance of displaced intimal calcium also favors an IMH over a classic aortic dissection [21].









