Location of the primary tumor (no. of patients with nodal metastasis)
Suprahilar
Hilar
Para-caval
Retro-caval
Interaorto-caval
Para-aortic
Common iliac
External iliac
Obturator
Internal iliac
Presacral
Right
RP (22)
–
14 (84 %)
8 (36 %)
9 (41 %)
3 (14 %)
–
–
–
–
–
UU (3)
–
1 (33 %)
–
1 (33 %)
2 (66 %)
–
–
–
–
–
MU (5)
–
–
–
1 (20 %)
4 (80 %)
–
–
–
–
–
LU (7)
–
–
–
–
–
–
4 (57 %)
1 (14 %)
5 (71 %)
2 (29 %)
1 (14 %)
Left
RP (25)
–
20 (80 %)
–
–
1 (4 %)
11 (44 %)
–
–
–
–
UU (0)
–
–
–
–
–
–
–
–
–
–
MU (5)
–
–
–
–
–
5 (100 %)
–
–
–
–
LU (8)
–
–
–
–
–
–
4 (50 %)
2 (25 %)
3 (38 %)
1 (13 %)
Assouad et al. reported on the lymphatic drainage from the kidney using adult cadavers [25]. The lymphatic flow from the right kidney travels through both the anterior and posterior aspect of the inferior vena cava whereas on the left it connects to the left renal hilar and/or the para-aortic nodes. The results from their basic studies are very similar to those of the mapping study in the clinical setting reported by Kondo.
Based on the findings from these two studies, Kondo et al. currently propose dissecting the regional lymph nodes within a defined anatomical extent in which the incidence of metastasis is in the 10 % or more range (Fig. 7.1) [24]. They recently reported the results of a prospective study confirming the survival benefit of lymphadenectomy in tumors of the renal pelvis using this anatomical template [26]. The rationale of the template for renal pelvic cancer appears to be supported by this study. However, this template needs to be validated by other studies examining lymph node mapping in UCUT.
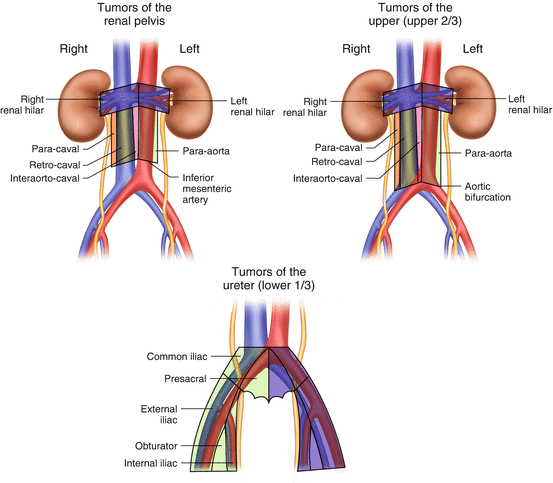

Fig. 7.1
Template of the anatomical lymph node dissection extent
Staging Benefits of Lymphadenectomy in UCUT
Improved staging is one of the expected advantages of lymphadenectomy. It may provide more accurate stratification of patients according to lymph node metastases status. In bladder cancer, the staging benefit of extending the template of lymphadenectomy has been thoroughly studied. The incidence of node-positive disease increases as the number of lymph nodes retrieved becomes higher by extending the cranial boundary of lymphadenectomy from below the bifurcation of the common iliac artery to that of the aortic bifurcation [14, 27–29]. Thus, extended lymphadenectomy is likely to improve the staging accuracy in bladder cancer although the optimal template remains debated and the extent of the benefit probably limited. Indeed a more thorough PLND and subsequently more LNs evaluated improve staging; however mapping data suggests that LN spread to the common arteries or above in the absence of LN involvement in the lower packets is a rare event, likely no more than 6–7 %.
In UCUT, Komatsu et al. examined the efficacy of a wide template of lymphadenectomy in the improvement of staging accuracy in 36 patients [20]. They used a wide template for lymphadenectomy similar to that proposed by Kondo and colleagues [24]. A clear stratification of patients with nodal involvement (pN+) was observed, with lower CSS than those without nodal metastases (pN0) [18]. Roscigno et al. reported that patients with pN0 confirmed by lymphadenectomy had a higher 5-year CSS than those without lymphadenectomy (pNx) (73 % versus 48 %, p < 0.001) [30]. They also used a similar template in their study. Five-year CSS did not significantly differ between patients with pNx and those with pN + (48 % versus 39 %, p = 0.476).
Results from several multi-institutional studies have been published, as shown in Table 7.2. Roscigno et al. collected data from 13 institutions and conducted a similar analysis, showing that the 5-year CSS increases incrementally from pN + to pNx to pN0 (35 % vs 69 % vs 71 %, p < 0.001 and p = 0.032) in an analysis of 1,130 patients with pT1 or higher [31]. This difference became more obvious when they analyzed 813 patients with pT2 stage or higher (5-year CSS: 33 vs 58 vs 70 %, p < 0.001 and p = 0.017). Abe et al. also showed that survival free from any recurrence, including locoregional or distant recurrences, was significantly higher in patients with pN0 than those with pNx in patients with pT2 or higher [32]. In contrast, there was no significant difference in any recurrence-free survival between pN0 and pNx in patients with pT1. In patients with advanced disease, these two multi-institutional studies showed significantly higher survival of patients with pN0 than those with pNx (Fig. 7.2).
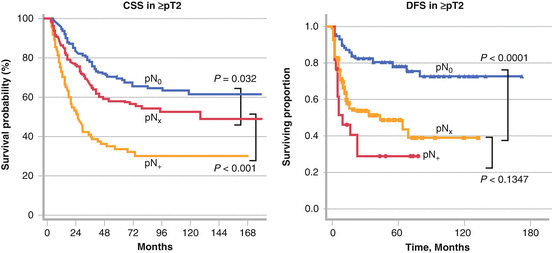
Table 7.2
Reports on staging benefit of lymphadenectomy in UCUT
Authors | Year | Institute | Subject | No. of patients | Template of LND | Results | Staging benefits | References |
|---|---|---|---|---|---|---|---|---|
Roscigno | 2008 | Single | ≥pT2 | 132 | Described | 5y-CSS: pN0 73 % > pNx 48 % (p < 0.001) = pN + 39 % (p = 0.476) | Yes | [30] |
Roscigno | 2009 | Multi | ≥pT1 | 1130 | Not well described | 5y-CSS: pN0 77 % > pNx 69 % (p = 0.032) > pN + 35 % (p < 0.001) | Yes | [31] |
≥pT2 | 813 | 5y-CSS: pN0 70 % > pNx 58 % (p = 0.017) > pN + 33 % (p < 0.001) | ||||||
Abe | 2010 | Multi | pT1 | 66 | Not well described | RFS: pN0 = pNx (p = 0.702) | Yes in ≥ pT2 | [32] |
≥pT2 | 227 | RFS: pN0 > pNx (p < 0.001) = pN + (p = 0.134) | ||||||
Burger | 2011 | Multi | Organ- confined | 519 | Not well described | CSS: pN0 = pNx = pN+ | Yes In locally advanced disease | [33] |
Locally advanced | 266 | CSS: pN0 = pNx (p = 0.633) > pN + (p < 0.001) | ||||||
Lughezzani | 2010 | Multi | pT1, pT2 | 1324 | Not described | CSS: T1 pN0 = pNx (p = 0.4) = pN + (p = 0.1) T2 pN0 = pNx (p = 0.8) = pN + (p = 0.1) | Yes In ≥ pT3 | [34] |
pT3, pT4 | 1382 | CSS: T3 pN0 = pNx (p = 0.9) > pN + (p < 0.001) T4 pN0 = pNx (p = 0.3) > pNx (p < 0.001) | ||||||
Mason | 2012 | Multi | All patients | 1029 | Not described | OS: pN0 66.1 % = pNx 66.0 % (p = 0.617) > pN + 22.3 % (p < 0.01) | Yes | [35] |
Ouzzane | 2013 | Multi | All patients | 714 | Not described | 5y-CSS: pN0 81 % = pNx 85 % (p = 0.6) >pN + 47 % (p < 0.001) | Yes but in T1 ? | [36] |
≥pT2 | 337 | CSS: pN0 = pNx (p = 0.44) = pN + (p < 0.15) |

Fig. 7.2
Staging benefit of lymphadenectomy by stratification of patients with pN0 vs pNx. Adapted from Roscigno. J Urol. 2009: 181:2482 and Abe. Eur J Surg Oncol. 2010: 36:1085
Other studies could not find an improvement in survival in patients with pN0, but reported worse survival rates in those with pN + than those with pNx. Burger et al. also examined the influence of lymph node status on patient survival in 785 patients from nine institutions [33]. They showed that lymphadenectomy can identify patients with pN + who have significantly lower CSS in both organ-confined disease and those with locally advanced disease. Stratification of the patients with pN0 in terms of survival from those with pNx was possible only in locally advanced disease. Lughezzani et al. conducted a population-based study by using a surveillance, epidemiology, and end results (SEER) database [34]. Lymphadenectomy could not discriminate between patients with pN0 and those with pNx at any stage of the disease. Both in uni- (HR: 1.19; p = .09) and multivariate analysis pN(x) vs pN(0) status was not associated with worse survival (HR: 0.99; p = .9). Mason et al. collected data from 1,029 patients treated in ten institutions in Canada [35]. The number of positive nodes and the number of nodes removed were not associated with survival although it provided more accurate staging. Ouzzane et al. reported the multi-institutional results of 714 patients in France [36]. A better stratification through lymphadenectomy in patients with pN + was observed, but not in those with pN0 when they analyzed all patients. In contrast to other studies, CSS was not significantly different among these three groups in patients with pT2 or higher. It is unclear whether their results mean that a staging benefit is observed in patients with pT1 or lower. Representative results from Lughezzani et al. and Bergur et al. are shown in Fig. 7.3.
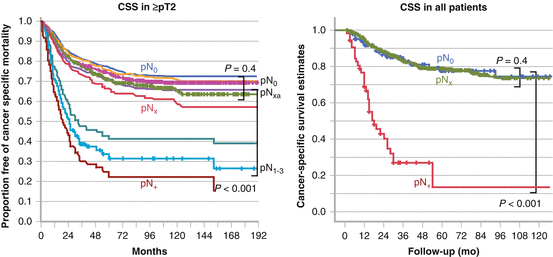

Fig. 7.3
Staging benefit of lymphadenectomy by stratification of patients with pN + vs pNx. Adapted from Lughezzani. Urology. 2010: 75:118 and Burger. World J Urol. 2011: 29:465
When summarizing these results in Table 7.2, two important points should be emphasized. The first is that all reports support a staging benefit. The results can be divided into two categories: those reporting a better patient stratification of pN0 from pNx, and those stratifying only pN + but not pN0 from pNx. In the studies reporting the latter results, the extent of lymphadenectomy was not described. The extent of lymphadenectomy may have influenced their results, but this remains to be determined. The second point is that this staging benefit appears to be more prominent in patients with muscle-invasive disease. Guidelines currently support a role for lymphadenectomy in the optimal staging of high-grade UCUT [37, 38]. However, it remains unknown what the minimally required extent of lymphadenectomy is for adequate staging purposes since the template of lymphadenectomy was not standardized in most of these studies. Of note, Xylinas et al. developed a pathological nodal staging model that allows quantification of the likelihood that a patient with pathologically node-negative disease has, indeed, no lymph node metastasis. This is the prediction of the true nodal status in patients with pathological lymph node-negative upper tract urothelial carcinoma at radical nephroureterectomy. The probability of missing lymph node metastasis decreased as the number of nodes examined increased. Even when five nodes were examined, 12 % of patients would have been misclassified. The proportion of those with a positive node increased with advancing pathological T stage and lymphovascular invasion [39].
Therapeutic Benefits of Lymphadenectomy in UCUT
In contrast to the staging benefit supported by most of the studies, the role of lymphadenectomy in UCUT in improving patient survival still remains controversial. One reason behind the difficulties in proving the therapeutic benefits of lymphadenectomy is that in patients with micro-metastatic disease captured by lymph node dissection, a major part of the benefit is likely driven by adjuvant chemotherapy, not necessarily the surgical removal of the nodes themselves, which serves as a staging procedure. Extrapolating evidence obtained from experience with multi-modal therapy of patients with urothelial bladder cancer, additional improvements in oncological outcomes for patients with UTUC are likely to be achieved through integration of effective systemic chemotherapy in addition to the local control and staging provided by surgery [40]. In addition, especially for patients suffering from advanced tumor stages, survival rates have not dramatically improved over the last decades with distant metastases being one of the main reasons for treatment failure. Chemotherapy in an adjuvant or neoadjuvant setting seems therefore to be a promising approach worth exploring which may further blur the true survival benefit of lymphadenectomy in UTUC [41].
Several studies from single institutions have suggested an improvement in patient survival by performing lymphadenectomy.
Kondo et al. compared patient survival according to lymphadenectomy status [42]. They first determined that regional node metastases were observed in 30 % or more of patients based on their mapping study [23]. They divided their retrospective cohort into three subgroups, including patients for whom the regional nodes were all dissected (complete lymphadenectomy (CompLND)); those in whom lymphadenectomy did not include all regional sites (incomplete lymphadenectomy (IncompLND)); and those without lymphadenectomy (No-LND). CSS was not significantly different among the three groups when all patients were included. But the survival rate increased incrementally from No-LND to IncompLND to CompLND in 88 patients with pT3 or higher (Fig. 7.4). The difference in CSS between CompLND and No-LND was statistically significant. Moreover, CompLND was found to be a significant independent factor to reduce the risk of cancer-specific mortality. They conducted their revised analysis by increasing the number of the patients to 191 with pT2 or higher and 140 with pT3 or higher [24]. CompLND significantly improved CSS compared to IncompLND or No-LND in patients with pT2 or higher (p = 0.03) and in those with pT3 or higher (p = 0.01) (Fig. 7.4). Brausi et al. examined the influence of lymphadenectomy on a relatively wide scale in 82 patients with pT2 or higher [43]. The extent of lymphadenectomy they used was as follows: the para-aorta or para-caval between the renal hilus and the inferior mesenteric artery for renal pelvis or upper ureteric cancers; the para-aorta or para-caval, between the renal hilus and bifurcation of the common iliac artery for mid-ureteric cancers; and the pelvic nodes on the ipsilateral side for lower ureteric cancers. The disease-specific survival (DFS) of 40 patients with lymphadenectomy was higher than in those with No-LND (81.6 % versus 44.8 %, p = 0.007) (Fig. 7.5). They also showed LND as a significant independent factor to reduce the risk of cancer-specific mortality. It should be mentioned though that these studies were non-randomized, therefore preventing strong and definitive conclusions. Roscigno et al. conducted a similar study in the retrospective cohort of 132 patients with pT2 or higher [30, 44]. The extent of lymphadenectomy was also similar to that used in the study by Brausi [43]. Lymphadenectomy significantly improved CSS of the patients compared to those with No-LND (5 year CSS: 57 % versus 40 %, p = 0.01). They also showed a higher 5-year CSS in patients with pN0 than in those with pNx (72 % versus 39 %, p < 0.001), emphasizing the prognostic disadvantage of omitting lymphadenectomy (Figure 7.5). Multivariate analysis showed that lymphadenectomy and pN0 status were significant independent predictors for CSS.
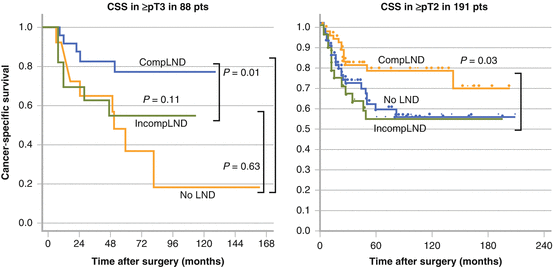
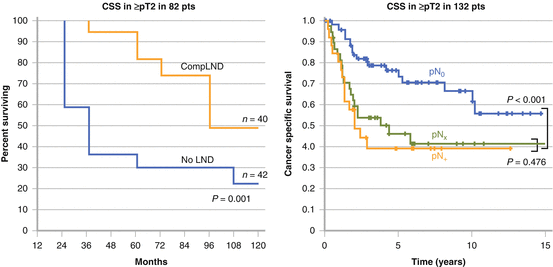

Fig. 7.4
Cancer-specific survival according to the status of lymphadenectomy. Adapted from Kondo. J Urol. 2007: 178:1212 and Kondo. Int J Urol. 2012: 19:710

Fig. 7.5
Survival benefit of lymphadenectomy in advanced disease. Adapted from Brausi. Eur Urol. 2007: 52:1414 and Roscigno. Eur Urol. 2008: 53:794
Several multi-institutional studies have been published on this very topic with diverging results. Multi-institutional studies have an advantage in that the results are confirmed with a large number of patients. On the other hand, the drawback is the lack of standardization of the extent of lymphadenectomy. Therefore results should be interpreted with caution. The results of a study involving 1,130 patients from 13 international institutes reported by Roscigno showed no therapeutic benefit from LND. Five-year CSS did not significantly differ between the patients with LND and those without (66 % vs 69 %, p = 0.23) [31]. They further examined the influence of the extent of lymphadenectomy on patient survival in patients with ≥ pT1pN0 using the same data set [44]. In 552 patients who underwent lymphadenectomy, CSS was significantly higher in patients for whom 8 or more LNs were removed than for those with an LN yield of less than 8 (84 % versus 73 %, p = 0.038), and the number of LNs removed independently influenced the CSS of patients (Fig. 7.6). This could also represent stage migration due to improved LN staging and not necessarily be related to a real therapeutic benefit.
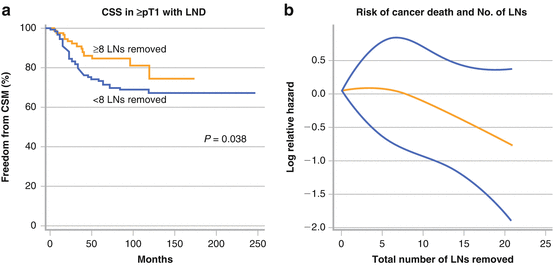

Fig. 7.6
Influence of LND extent influencing on patient survival. Adapted from Roscigno. Eur Urol. 2009: 56:512
In addition, the risk of cancer-specific mortality continued to decrease as the number of the lymph nodes removed increased (Fig. 7.6).
Abe et al. also examined the influence of lymph node status in 293 patients from multiple institutions [32]. These authors reported a staging benefit of lymphadenectomy in patients with pT2 or higher by showing an improved CSS in patients with pN0 than those with pNx (Fig. 7.2). They also performed multivariate analyses and showed that pNx was an adverse factor not only for locoregional recurrence (HR 3.96, p = 0.0026), but also for distant relapse recurrence (HR 2.86, p = 0.0024). The study by Burger also demonstrated that patients with pN0 disease presented with a lower risk of recurrence (HR 0.3, p < 0.001) and death (HR: 0.3; p < 0.001) compared to pNx disease on multivariate analysis in locally advanced disease [33]. It should be mentioned though that these results should be interpreted with caution as there is no proof a better staging translate into improved survival.
In contrast to the abovementioned studies, other multi-institutional studies failed to support a therapeutic role for lymphadenectomy. Lughezzani reported the results of a population-based study of 2,824 patients in the SEER database [34]. They failed to show any therapeutic benefit of lymphadenectomy. No difference in CSS between pN0 and pNx even in patients with muscle-invasive disease (p = 0.4) was reported (Fig. 7.3). Multivariate analysis also showed that omitting lymphadenectomy (pNx) had no influence on cancer-specific mortality. Similar results were reported by Ouzzane from 786 patients in a French multi-institutional study [36] and by Mason from 1,029 patients in Canada [35]. The latter two studies showed no difference between patients with pN0 and those with pNx, and pNx did not reduce the risk of cancer-specific mortality after adjusting for other clinical parameters by multivariate analysis. Again, the extent of lymphadenectomy was not described in the latter studies due to the nature of the multi-institutional studies.
The jury is therefore still out and it should be outlined that improved staging does not mean therapeutic advantage at the present time. All these above studies were retrospective, but one prospective study was recently reported. Kondo and colleagues conducted a single-arm prospective study at two Japanese institutes [26]. Template-based lymphadenectomy was performed according to their previous mapping study. Patients who underwent complete lymphadenectomy in the prospective setting were designated as Pros-CompLND, and complete lymphadenectomy in the retrospective cohort as Retro-CompLND. The control group included patients who underwent lymphadenectomy in the retrospective cohort and those without lymphadenectomy. In patients with renal pelvic cancer stage pT2N0M0 or higher, the 3-year CSS was 90.8 % in Retro-CompLND, 87.9 % in Pros-CompLND, and 64.7 % in No-LND (Fig. 7.7). Patients with Pros-CompLND had significantly higher survival rates than the No-LND groups (p = 0.04). Multivariate analysis supported that complete lymphadenectomy is a significant independent factor to reduce the risk of cancer-specific mortality. They concluded that lymphadenectomy is strongly recommended for patients with renal pelvic cancer of ≥ pT2. In addition, their template of lymphadenectomy used for renal pelvic cancer was further validated by this prospective study. On the other hand, this therapeutic benefit could not be observed in patients with ureteral cancer even when limited to muscle-invasive disease (Fig. 7.7), the reason for which is unclear.
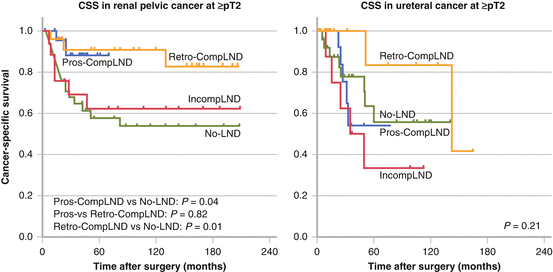

Fig. 7.7
Results from a prospective study on CSS comparing no LND to incomplete and extended LND. Adapted from Kondo T, Hara I, Takagi T, et al. Therapeutic benefit from template-based lymphadenectomy in urothelial carcinoma of the renal pelvis: A prospective study. Int J Urol. 2014: 21:453-9
A limitation is that those patients without LN dissection are by definition Nx with many N + in reality and thus falsely classified. These patients may have a worse outcome because they are understaged, and not necessarily because the LN dissection was not completed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






