Fig. 6.1
Mid rectal annular tumour spreads beyond the muscularis propria up to 8 mm (a– yellow arrows). Mesorectal fascia (b – red line, which defines the mesorectum) is involved by direct spread of tumour (distance from the tumuor tomesorectal fascia is less than 1 mm – CRM +)
The staging of recurrent disease is more complex. The prognostic factors which influence survival outcomes in primary disease do not fully apply. Other considerations, mainly the extent and pattern of local recurrence are most important in treatment planning and to accurately delineate the extent of disease a combination of imaging modalities may be used [27].
Imaging-Based Risk-Stratification
As mentioned above, not all patients require pre-operative treatment – this is determined by accurate risk-stratification. Depending on the local policy, risk-stratification will be based on the identification of specific features. Stratification can take place on initial presentation whereby traditional prognostic factors based on histopathological studies in primary tumours are used as a basis – tumour depth (T-stage), nodal disease (N-stage), extramural venous invasion (EMVI). Further stratification can be performed following CRT however the influence on traditional prognostic factors is unclear in this situation.
Tumours which exhibit evidence on initial staging of CRM involvement, tumour penetration beyond 5 mm into the mesorectum (T3c), venous invasion, N2 disease are considered high-risk and will routinely be offered neo-adjuvant treatment. These features are accurately identified on serial MRI and have been shown to correlate well with pathology and overall survival outcomes [28–33].
MRI Technique
For optimal results using MRI it is essential to adhere to specific technical criteria [34, 35]. This includes correct field of view (FOV), field alignment and sequences. Incorrect use of any of these technical considerations can results in under- or over-staging of disease and consequently sub-optimal management. For example, using an inadequate FOV can make accurate delineation of the important anatomical structures difficult. Using correct FOV also affects the voxel size. If the voxel size is increased, resolution is lost and morphological characteristics become less obvious. Incorrect FOV is the most common error that leads to poor-quality images. Figure 6.2a shows the MRI of a patient with a prominent node in the mesorectum. Distinguishing whether this is a malignant or benign node is challenging if the resolution is poor and in this example it is difficult to adequately visualise the nodal architecture – FOV is 22 cm × 22 cm. In Fig. 6.2b, the correct FOV is used (16 × 16 cm) and it is much more straightforward to delineate the nodal anatomy.
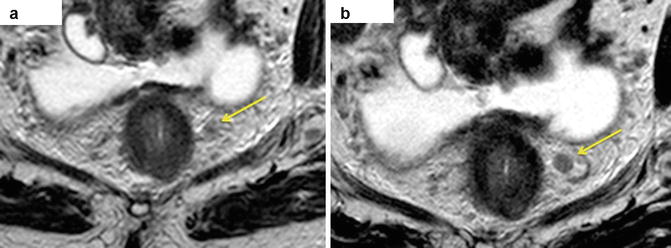

Fig. 6.2
Differences in FOV for imaging nodal disease. (a) Shows 22 cm × 22 cm FOV and consequent poor quality of image (lymph nodes are difficult to assess – yellow arrow). This is much improved in (b) where the FOV is 16 cm × 16 cm. The nodal anatomy is clearly visible with particular respect to the nodal border and signal characteristic (yellow arrow)
Another common mistake which results in inaccurate staging is incorrect field alignment. Sequences must be taken in the correct plane with respect to the long axis of the rectum. Typical images are taken in 3 mm slices. Figure 6.3 shows the correct field alignment based on the MERCURY protocol [20, 36]. If the field is incorrect, the tumour edge is inaccurately identified.
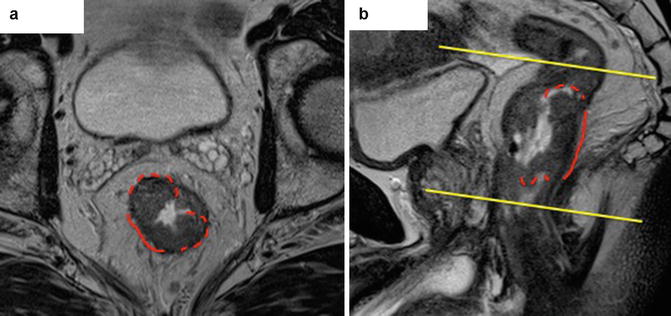

Fig. 6.3
Correct alignment of MRI field. This is perpendicular to the long axis of the rectum and commonly mistaken resulting in under- or overstaging of disease. Axial (a) scans should be taken perpendicular to the rectal wall on sagittal image (b) s at the level of invading tumour border (continuous red line) which is a distance between raised rolled edges (where submucosal layer is preserved – dashed red line)
Initial localization images in the coronal and sagittal planes are needed to plan further high-resolution images. The first series are T2-weighted sagittal, turbo spin-echo sequences from one pelvic sidewall to the other enable identification of the tumour. The second series consists of large-field-of- view axial sections of the whole pelvis. The third series consists of the high-resolution images that are T2-weighted thin-section axial images through the rectal cancer and adjacent tissues.
Prognostic Factors in Primary Rectal Cancer
Accurate identification of the important prognostic factors is the main role of MRI in the local staging of disease. The following section describes the evidence for these factors and the accuracy of detection.
Tumour Depth
The progression of disease and characteristic spread of rectal cancer is through the layers of the bowel wall. The micro-structure of the bowel wall can be identified on MRI which fits with the traditional TNM staging system [37]. Spread of tumour through the bowel into the surrounding mesorectum and beyond is associated with worsening prognosis. The risk of recurrence for T1, T2 and T3 tumours independent of lymph node involvement are in the order of 5, 10, and 25 %, respectively. However there is a distinct cut-off in terms of prognosis relating to the depth of penetration. Tumours which only minimally extend into the mesorectum and those which are confined to the bowel wall are considered to be ‘good prognosis’ or ‘low-risk’ cancers. These can be managed with primary surgery providing there are no other adverse features.
Accurate assessment of this relies on the identification of the layers of the bowel wall which is accurately accomplished by both MRI and EAUS. EAUS may be more useful for T1 and T2 tumours where accurate identification of the mesorectal fascia has less importance. EAUS has increased accuracy for defining the detail of the bowel wall structure which is particularly useful when planning mucosal resection or transanal excision. Sensitivity and specificity for T1 cancers is 87.8 and 98.3 %, respectively [38]. As transanal endoscopic microsurgery (TEMS) and endoscopic submucosal resections become more popular, greater detail of the bowel wall is essential to select appropriate patients.
MRI can readily identify the layers of mucosa and muscle through distinct signal characteristics. T2-weighted images are particularly useful for this. The mucosal layer is seen as a very fine line of low signal intensity overlying the much thicker and higher signal of the submucosa. Outside this the muscularis propria can be seen as a duel-layer representing the inner circular and the outer longitudinal muscle layers. The latter has a typically irregular appearance due to vessels traversing the rectal wall. The perirectal fat is identified as a high signal with signal void areas surrounding the relatively low signal intensity of the muscularis. This is all enveloped by the fine layer of low signal intensity representing the mesorectal fascia.
To understand the prognostic relevance of spread into the mesorectum it is important to appreciate its unique nature. The rectum is the only part of the gastrointestinal tract which is intimately surrounded by a distinct mesentery containing lymphovascular structures. This fatty layer can be readily seen in-vivo although it is not so easily identifiable in the cadaver. The outermost boundary is defined by the mesorectal fascia (MRF) which demarcates the CRM during surgical excision. The CRM acts an oncological barrier to tumour spread. However increasing penetration into the mesorectum (T3 disease) is associated with increasing rates of disease recurrence [39–45].
Cawthorn, Merkel and Willett were the first to report on this heterogeneity within T3 tumours. Cawthorn reported 5 year survival to be 55 % with tumour penetration less than 4 mm into the mesorectum compared to 25 % when more than 4 mm [40]. Merkel studied patient’s survival characteristics with T3 tumours and used a cut-off of 5 mm. Those patients with extramural spread of more than 5 mm had 5 years survival rate of 54 % compared with 85 % for those patients whose tumours had extramural spread of less than 5 mm [46]. These results were independent of lymph node involvement. These early studies highlight the importance of accurate measurement of tumour penetration into the mesorectum and those tumours with a worse prognosis, namely T3c and T3d. Therefore the distinction between T2 and T3 tumours with less than 5 mm mesorectal spread – T3a and T3b; becomes irrelevant as these patients will have minimal benefit from CRT. This has led to the sub-staging of T3 tumours which has been adopted by the UICC TNM classification since 1993 (Fig. 6.4).
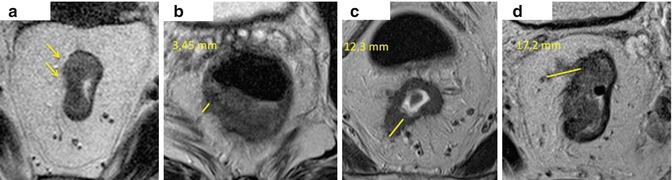

Fig. 6.4
T3 sub-classification based on penetration into the mesorectum. T3a (a) –initial tumour spread into the mesorectum (1 mm – yellow arrows), the muscularis propria is not preserved. T3b (b) – tumour spread beyond the muscularis measures 3 mm (yellow line).T3c (c) – tumour spread measures 12 mm. T3d (d) – tumour spread is more then 15 mm (17 mm)
More recently, the prognostic importance of T3 substage has been recognised following CRT. Merkel et al. studied the prognostic impact on survival outcomes for patients with T3a and T3b tumours (ypT3a/b) [47]. They found that ypT3 subclassification was an independent prognostic factor for disease-free, observed and cancer-related survival [48]. ymrT3 sub-classification has also been found to predict prognosis in terms of overall and disease-free survival with local recurrence rates to be less than 4 % in the MRI-predicted ‘good tumours’ [28].
MRI has been shown as the optimal modality for identifying the CRM and mesorectum. In addition to being able to detect the depth of tumour spread into the mesorectum to within 1 mm it can also identify the tumour edge with similar detail. . It has been shown to be able to identify potential tumour at the CRM to within 1 mm [19, 23, 49, 50]. Pathologists recognise a clear margin for tumour excision to be 1 mm. If tumour is seen within 1 mm of the CRM, it is said to be a ‘positive margin’ or ‘R1 resection’. In the MERCURY Study, a total of 349 patients underwent pre-operative MRI assessment followed by TME surgery were predicted to have clear margins. 327 (94 %) patients were subsequently found to have clear margins on histopathology [20]. This gave a specificity of 92 %. Taylor et al. have shown that rates of local recurrence decreased from 53 % with tumour less than 1 mm from the potential CRM to less than 8 % when the tumour distance from the mesorectal fascia was between 1 and 5 mm [49, 51]. A measured distance of 5 mm on MRI has been shown to strongly correlate with negative CRM on histology, which led to patients being offered chemoradiotherapy when tumours are within 5 mm of the mesorectal fascia. However, this results in substantial overt-treatment of patients with safe margins.
Nodal Disease – N Staging
The importance of solitary lymph node involvement in rectal cancer is now being challenged with respect to its risk of local recurrence [52]. Traditional teaching has suggested that malignant mesorectal lymph nodes are associated with local recurrence thus patients should be offered radiation therapy. The results of MRC CR07 trial seemed to give this further credence however these results did not account for sub-optimal surgery [14]. TME is the accepted surgical technique for rectal cancer and should be considered the single-most important factor in reducing local recurrence in the last century. Historical trials included patients who had undergone a wide variation of quality in their surgery and when the results of CR07 are taken with respect to which plane of resection was used, the story is rather different. The actual benefit for patients who have undergone TME surgery is less than 4 %. Therefore in the current modern era of rectal cancer management where high-quality precision surgery is the expected norm, it does not make sense to put patients through an intensive radiotherapy regime with minimal benefit. This is not to say that nodal disease does not have a bearing on metastatic disease but this can be treated with adjuvant systemic therapy rather than local radiotherapy. One must also remember that whilst neoadjuvant treatment improves local recurrence rates, it has no effect on overall survival.
Yet despite this, and whilst nodal involvement remains a consideration for units, correct technique and staging of nodes is paramount to avoid unnecessary treatment. The same basic technical principle apply but the criteria used to distinguish benign from malignant nodes is equally important. There has been a predilection for using size criteria when determining the nature of mesorectal nodes; that is, the larger the node, the more likely it will be malignant. There has been no robust trial evidence behind this or pathological correlation. A study which matched nodes from in vivo and specimen MRIs with pathology specimens showed that there was no useful size cut-off for predicting nodal status [53]. Further, a histological survey of over 12,000 lymph nodes in rectal cancer showed considerable size overlap between normal or reactive nodes and those containing metastases [54]. A perceived limitation of MRI is the lack of accuracy and ability to detect nodes smaller than 3 mm. Yet this may not be as clinically relevant as first appears. Only 2 % of nodes which are malignant were of this size [53].
More important than size is nodal border and the tumour signal within the node. when a high resolution MRI technique is used, it is easier to evaluate lymph node architecture and has enabled new criteria for lymph node involvement to be developed. Tumour infiltration into lymph nodes leads to characteristics radiological features which can be readily identified on MRI (Fig. 6.5). Tumour leads to capsular disruption causing the nodal border to become irregular as opposed to the more rounded border of benign nodes. A very small number of lymph nodes with a smooth bordered contour (<6 %) have been shown to be malignant whilst those demonstrating irregular outline are malignant in over 90 % of cases. Mixed signal intensity occurs due to the heterogeneity of the tumour and necrosis within the node. When using the signal characteristics and border outline together, the sensitivity is much improved. Using features of nodal border, contour and differing signal characteristics the sensitivity and specificity increases to 85 and 97 %, respectively. Endoanal ultrasound (EAUS) does not predict lymph node involvement any better. Indeed sensitivity and specificity for detection of cancerous lymph nodes in rectal cancer is 73.2 and 75.8 %, respectively [55] although more likely to be accurate in the more proximal parts of the rectum. Swollen reactive nodes, small blood vessels and even local structure such as the seminal vesicles may mimic malignant nodes.
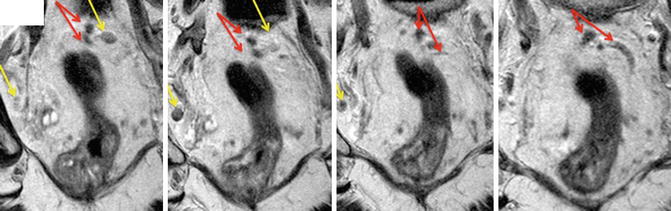

Fig. 6.5
MRI showing mesorectal nodes. Both mesorectal and pelvic nodes are round/oval structures (yellow arrows), that usually seen on not more than 2–3 consecutive images. The operator must scroll through images so not to confuse them with vessels (red arrows)
Using the same criteria as above with T2-weighted MRI, lymph nodes can be accurately identified following neoadjuvant treatment. Koh et al. prospectively evaluated the MR staging of lymph nodes before and after chemoradiotherapy and compared this with histopatholgical analysis to demonstrate significant correlation between post-treatment MR assessment and histopathology of nodal disease [56].
Extramural Venous Invasion – EMVI
The prognostic effect of vascular invasion has been suspected for several decades however there has been huge variability in practice with regards to treatment decisions. Over recent years, there has been a refinement in the definition of vascular invasion in respect to colorectal cancer and, in particular, rectal cancer. This has been based on seminal work by Talbot in 1980 who demonstrated the importance of distinguishing extramural from intramural venous invasion [57, 58]. Extramural venous invasion (EMVI) is defined by evidence of tumour cells or, in the case of the radiological definition, tumour signal in the vasculature outside the muscularis propria. This means that it is found in more locally advanced tumour – T3 and T4 disease.
One of the main reasons behind the variability in treatment decisions when EMVI is present must be due to the heterogeneity in the literature. The historical studies which have been the basis of much of our current understanding in rectal cancer have used a variety of definitions in the methodology for sampling and analysis. In addition, there has been little detail in the techniques used for detection which has ultimately resulted in a wide range in prevalence [59–62]. Standardisation in reporting techniques have made data reporting in both pathology and radiology more accurate as EMVI is specifically sought [35, 63, 64].
The use of radiological detection of EMVI has helped drive its prognostic importance. It is considered in the MRI reporting sets of almost all prospective trials involving rectal cancer. EMVI has been investigated in patients undergoing primary surgery and in those which have undergone pre-operative treatment. One study investigated the rate of detection of EMVI on MRI (mrEMVI) compared with so-called Gold standard of pathology detection from the assessment of resection specimens [19]. Eighteen patients had large vessel EMVI visible on H and E stain. Fifteen of these 18 cases found mrEMVI. However more subtle involvement of tumour within smaller vessels was not resolved on MRI.
The radiological characteristics have been previously described (Fig. 6.6) [65]. To accurately identify EMVI on MRI, it is imperative to have a sound understanding and appreciation of the vasculature around the rectum. This can help distinguish venous disease from nodal deposits and it is necessary to ‘chase’ the signal along the length of the vessel through multiple images. Veins around the rectum are recognised on T2-weighted images as serpiginous or tortuous linear structures. Differentiating between larger and smaller vessels can be difficult and requires a combination of signal characteristics and morphology. The larger, named vessels such as the superior and middle rectal veins appear with anatomical consistency which helps in confident identification. Ideal assessment of mrEMVI must include the following: pattern of tumour margin (extension into small veins may produce a nodular border); location of tumour relative to major vessels; vessel calibre (tumour causes vessel expansion and increase in tumour signal in the lumen); and vessel border. Smaller venules can be seen perforating the normal outer rectal wall and produce a low to intermediate signal intensity in tubular structures on T2-weighted images.
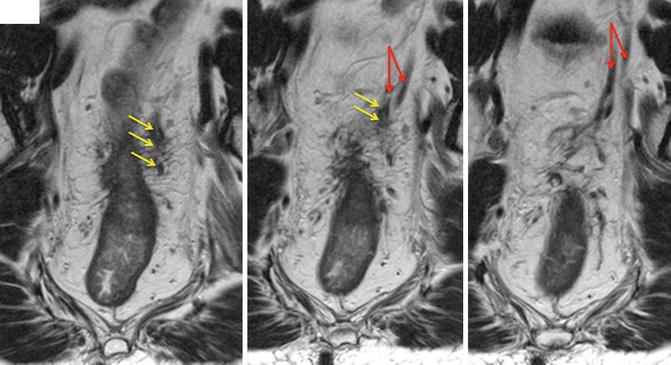

Fig. 6.6
EMVI shown in locally advanced tumour. The tumour signal can be seen extending into the veins and expanding it (red arrows) outside the bowel wall. This is characteristic of venous invasion. However, the operator must follow the signal through several images to ensure venous disease rather than nodal spread (yellow arrows)
Using the radiological characteristics, Smith et al. offered a scoring system to stratify the degree of venous invasion. A study of 142 patients investigated the accuracy of detection and prognostic relevance of mrEMVI [66]. Patients included in the study undergoing either primary TME-surgery (n = 94) or neo-adjuvant therapy followed by surgery (n = 48). The incidence of mrEMVI was initially 39.4 % but fell to 24.1 % for those patients who were re-imaged following pre-operative treatment – indicating a degree of ‘down-staging’. Recurrence free survival at 3 years was compared between mr- and histology-detected EMVI and reported as 35 and 34 %, respectively. Recurrence-free survival when EMVI was not present was 73.8 and 74.1 % respectively.
More recently, the effect of pre-operative chemoradiotherapy (CRT) on mrEMVI has been examined. Using the same radiological criteria as described above, EMVI can be re-assessed following CRT (Fig. 6.7). The radiological detection of EMVI using MRI has been compared with pathology-detected EMVI in patients following pre-operative treatment with long-course chemoradiotherapy [yu/chand/patel]. There has been concern by pathologists that the fibrosis which occurs following radiotherapy makes detection more difficult [67, 68]. This issue has been cited by some with respect to MRI identification of prognostic factors following CRT, however there reproducibility and accuracy of detection once appropriate training has been undertaken as reflected in the consistency of reporting within large collaborative studies such as MERCURY. A recent study has shown the advantage of using MRI rather than pathology for detecting EMVI after CRT (ymrEMVI versus ypEMVI) [33]. In the cohort of 188 patients, EMVI was detected in 99 patients after CRT when MRI criteria was used. On final pathology staging, only 36 patients were identified with EMVI. This would indicate that either MRI was over-estimating or pathology under-estimating EMVI. Yet this survival analysis revealed that by using either technique, evidence of EMVI led to worse disease-free survival at 3 years. This implied that ymrEMVI is either a unique prognostic phenomenon which is not directly comparable to ypEMVI or that ymrEMVI is more accurate in detecting EMVI following CRT.
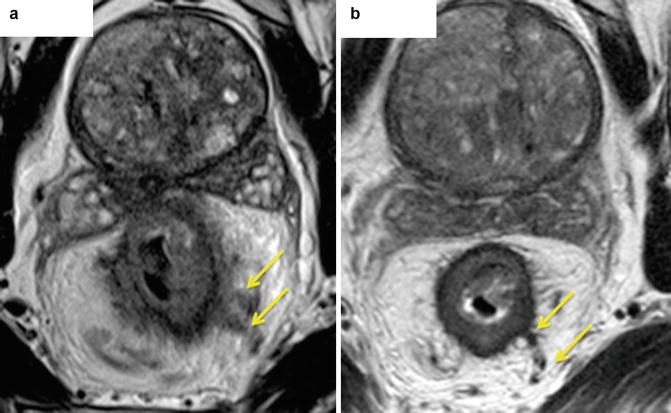

Fig. 6.7
Radiological features of mrEMVI after chemoradiotherapy (ymrEMVI). Fibrosis can be seen in the veins outside the bowel wall. Mid rectal annular tumour. Before CRT (a) – a gross expansion of extramural veins in present (yellow arrows), post CRT (b) – vein have the normal diameter with low signal intensity within them (yellow arrows), suggestive of fibrosis
EMVI can also be graded with a MRI-based tumour regression score. mrEMVI status has been shown to convert following CRT, that is, patients who are initially diagnosed with mrEMVI positive status can become mrEMVI negative [69]. This is accompanied with an improvement in disease-free survival. The degree of improvement in EMVI following CRT can be linked with a respective improvement in survival outcomes. In one study, where mrEMVI had regressed by 50 % of more the 3-year DFS was 87.8 % with a recurrence rate of only 9 %. Those patients that showed less than 50 % fibrosis had 3-year DFS 45.8 % with 44 % recurrence rate. This equated to a hazard ratio of 5.75. This introduces the concept of mrEMVI as an imaging biomarker [70].
In the context of post-CRT prognosis, there is a lack of robust prospective evidence to demonstrate which factors may be considered the most relevant. We continue to rely on the historical studies of patients that had undergone primary surgery and, in some cases, oncologically inadequate surgery with poor resection planes achieved. Recently, EMVI has been shown to confer a worse prognosis in terms of disease-free survival in rectal cancer patients who have undergone pre-operative long-course CRT than nodal disease. A study which examined the survival outcomes of patients with stage II and III disease found that patients with stage II disease and MRI evidence of EMVI had similar outcomes to those patients with stage III disease. Further, patients with stage III tumours had worse disease-free survival when there was evidence of EMVI [71].
Current multicentre studies such as BACCHUS (Bevacizumab And Combination Chemotherapy in rectal cancer Until Surgery) and MARVEL (Molecular And Radiological EValuation of Extramural venous invasion in RectaL Cancer) may help further understand the true importance of EMVI.
Height of Tumour
The position of height of a rectal cancer is measured from the anal verge. Although there are differing definitions of the exact length of the rectum it is the importance of ‘low tumours’ which must be considered. Tumours found within the distal 6 cm are classified as “low” rectal cancers. Low tumours are associated with local recurrence and anastomotic leak following surgery [72].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






