The Relevance of Sorbent Technology Today
Jose A. Diaz-Buxo
Stephen A. Merchant
David Updyke
Susan E. Bentley
On average, conventional dialysis machines process 30 to 50 L/hr or 100 to 200 L per session of dialysis solution made from highly purified water. In contrast, sorbent dialysis requires as little as 6 L of potable tap water to produce and regenerate high-quality dialysate for an entire treatment. With sorbent dialysis, spent dialysate from the dialyzer is not discarded to a drain but is regenerated by passing it through a sorbent cartridge. The layers of compounds in the cartridge take advantage of three basic principles of chemistry: carbon binding, enzyme conversion, and ion exchange, to remove uremic toxins and regenerate high-quality bicarbonate dialysate during a dialysis treatment.
Sorbent devices operate without being connected to a water supply or a drain; therefore, additional benefits are system mobility and the flexibility of treatment delivery in a wide range of environments. Sorbent systems have been used for acute dialysis in critical care units and at the patient bedside, for home hemodialysis, for military operations, for disaster relief, in rehabilitation centers and nursing homes, in remote locations, and to treat patients on vacation in remote locations. Without the need for plumbing installation or electrical modification, the potential treatment environments with sorbent systems are numerous.
Sorbent systems provide an opportunity to drive innovation, portability, flexibility, and miniaturization in the dialysis domain.
I. PRINCIPLES OF SORBENT DIALYSIS. In sorbent dialysis, the spent dialysate is continuously regenerated to form fresh dialysis solution by passing it through a sorbent cartridge. The initial dialysis solution is mixed in a designated jug using dry powders and 6 L or less of potable tap water. Prior to starting dialysis, this initial solution is recirculated through the sorbent cartridge for the purpose of removing contaminants. This initial recirculation slightly alters the starting dialysis solution’s electrolyte composition, as described in more detail below. Once dialysis has been initiated and the patient has been connected into the system, the “spent” dialysate is then routed from the dialyzer outlet port through the sorbent cartridge. In the cartridge, metabolic waste products dissolved in the spent dialysate are adsorbed and/or exchanged for
sodium, hydrogen, and bicarbonate ions. The sorbent cartridge also removes potassium, calcium, and magnesium. Regeneration of the final dialysate solution is completed when potassium, calcium, and magnesium are added to the dialysis solution exiting the cartridge by an infusion pump.
sodium, hydrogen, and bicarbonate ions. The sorbent cartridge also removes potassium, calcium, and magnesium. Regeneration of the final dialysate solution is completed when potassium, calcium, and magnesium are added to the dialysis solution exiting the cartridge by an infusion pump.
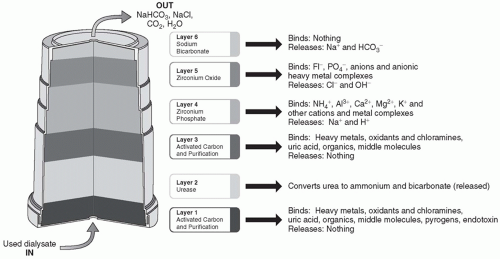
A. The sorbent cartridge. The sorbent cartridge (Fig. 19.1) consists of six layers of materials, which are designed to remove contaminants and uremic solutes while at the same time maintaining an appropriate dialysate composition. Spent dialysate flows through the cartridge from bottom to top. The first and third layers with which the dialysate comes into contact contain activated carbon. These layers adsorb heavy metals, chloramines, and other contaminants that can be found in the tap water. In addition, the activated carbon adsorbs many of the organic and middle molecule uremic solutes found in spent dialysate, including creatinine and uric acid. The second layer is an enzyme-retention layer. The enzyme present is urease, which catalyzes the conversion of urea to ammonium bicarbonate. The fourth layer contains zirconium phosphate and is a cation exchange layer. Its primary function is to adsorb the ammonium ion generated by urea hydrolysis that took place in the second layer. In addition, this cation exchange material adsorbs other positively charged species such as magnesium, calcium, and potassium, as well as heavy metal cations that may be found in tap water such as copper and iron. In exchange for the adsorbed cations, the zirconium phosphate releases hydrogen and sodium. The fifth layer is an anion exchange layer containing zirconium oxide. This material adsorbs phosphate, fluoride, and other anions, such as oxoanions of heavy metals, and in exchange release chloride and hydroxyl anions. The sixth layer contains sodium bicarbonate. It does not bind anything but releases sodium and bicarbonate.
B. Removal of contaminants from the prime solution during predialysis recirculation through the sorbent cartridge. The initial dialysis solution or “prime” is made by combining dry chemicals with 6 L or less of municipal tap water. This tap water must meet EPA drinking water standards. This initial mixture is not suitable as a dialysis solution, as it may contain contaminants. However, a brief predialysis recirculation of the prime through the sorbent cartridge removes almost all contaminants normally present in potable tap water (assuming that it contains no more than the maximum allowable contaminant limits [MA-CLs] for potable water as indicated by the US EPA) to levels required for dialysis solution as indicated by ANSI/AAMI RD52. There are two exceptions: recirculation of the prime through the sorbent cartridge does not remove sulfate or nitrate to an appreciable extent. However, as long as initial levels of sulfate and nitrate are less than the maximal allowed levels for tap water (10 mg/L for nitrate and 250 mg/L for sulfate), because
only 6 L of tap water are used, the total sulfate (or nitrate) load potentially transferable to the patient is small.
only 6 L of tap water are used, the total sulfate (or nitrate) load potentially transferable to the patient is small.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree