The Patient with Hyponatremia or Hypernatremia
Robert W. Schrier
Tomas Berl
Control of serum sodium and osmolality. Under physiologic conditions, the concentration of sodium in plasma is kept in a very narrow range, between 138 and 142 mEq/L, despite great variations in water intake. Because sodium is the predominant cation in extracellular fluid (ECF), this reflects the equally narrow range in which the tonicity (osmolality) of body fluids is regulated, between 280 and 290 mOsm/kg. Therefore, calculated plasma osmolality can be expressed as follows:
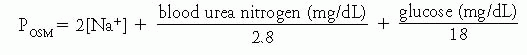
Serum sodium concentration and plasma osmolality are maintained in these normal ranges by the function of arginine vasopressin (AVP) and a very sensitive osmoreceptor that controls the secretion of this antidiuretic hormone. This hormone, in turn, is critical in determining water excretion by allowing urinary dilution in its absence and urinary concentration in its presence. Hyponatremic disorders supervene when water intake exceeds the patient’s renal diluting capacity. Conversely, hypernatremia supervenes in settings associated with renal concentrating defects accompanied by inadequate water intake.
Hyponatremia. Hyponatremia, defined as a plasma sodium concentration of less than 135 mEq/L, is a frequent occurrence in the hospitalized patient. It has been suggested that approximately 10% to 15% of patients in hospitals have a low plasma sodium concentration at some time during their stay. Hyponatremia in the ambulatory outpatient is a much less frequent occurrence and is usually associated with a chronic disease state.
I. INTERPRETATION OF THE SERUM SODIUM.
Under most clinical circumstances, a decrement in serum sodium reflects a hypo-osmolar state. However, in some settings a low sodium level could be associated with normal or even a high osmolality. The addition to the ECF of osmotically active solutes that do not readily penetrate into cells, such as glucose, mannitol, and glycine, causes water to move from cells to ECF, thereby leading to cellular water loss resulting in a decrement in serum sodium concentration. This translocational hyponatremia does not reflect changes in total body water (TBW), but rather the movement of water from the intracellular to the extracellular compartment.
In hyperglycemia, for each 100 mg/dL rise in blood glucose, a 1.6 mEq/L fall in plasma sodium concentration occurs as water moves out of cells into
the ECF. For example, in an untreated diabetic patient, as blood glucose rises from 200 to 1,200 mg/dL, the plasma sodium concentration is expected to fall from 140 to 124 mEq/L (1.6 mEq/L × 10 = 16 mEq) without a change in TBW and electrolytes. Conversely, treatment with insulin and lowering of the blood sugar from 1,200 to 200 mg/dL in this diabetic patient results in a comparable osmotic water movement from the ECF back into cells and a return of plasma sodium concentration to 140 mEq/L without any change in TBW.
the ECF. For example, in an untreated diabetic patient, as blood glucose rises from 200 to 1,200 mg/dL, the plasma sodium concentration is expected to fall from 140 to 124 mEq/L (1.6 mEq/L × 10 = 16 mEq) without a change in TBW and electrolytes. Conversely, treatment with insulin and lowering of the blood sugar from 1,200 to 200 mg/dL in this diabetic patient results in a comparable osmotic water movement from the ECF back into cells and a return of plasma sodium concentration to 140 mEq/L without any change in TBW.
Another setting in which hyponatremia can occur without a change in plasma osmolality is termed pseudohyponatremia. Pseudohyponatremia occurs when the solid phase of plasma, primarily lipids and proteins (usually 6% to 8%), is greatly increased, as in severe hypertriglyceridemia and paraproteinemic disorders. This falsely low reading is a consequence of the flame photometry methods that measure the concentration of Na+ in whole plasma and not only in the liquid phase. A measure of the true serum sodium can be obtained in undiluted serum analyzed with an ion-specific electrode that measures the concentration of sodium in serum water.
II. APPROACH TO THE HYPO-OSMOLAR HYPONATREMIC PATIENT.
In the absence of translocational hyponatremia or pseudohyponatremia, the most important initial step in the diagnosis of hyponatremia is an assessment of the ECF volume status.
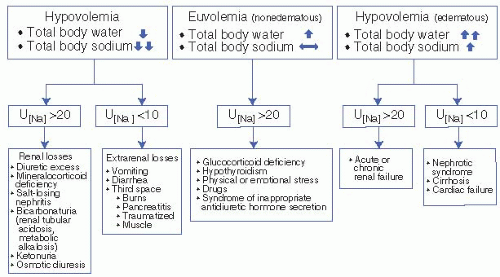
Sodium is the primary cation in the ECF compartment. Therefore, sodium, with its accompanying anions, dictates ECF osmolality and fluid volume. Hence, ECF volume provides the best index of total body exchangeable sodium. A careful physical examination focused on the evaluation of ECF volume status therefore allows for the classification of the hyponatremic patient into one of three categories: (a) hyponatremia in the presence of an excess of total body sodium (hypervolemic hyponatremia), (b) hyponatremia in the presence of a deficit of total body sodium (hypovolemic hyponatremia), and (c) hyponatremia with a near-normal total body sodium (euvolemic hyponatremia). For example, the edematous patient is classified as having hyponatremia with an excess of total body sodium. The volume-depleted patient with flat neck veins, decreased skin turgor, dry mucous membranes, and orthostatic hypotension and tachycardia is classified as having hyponatremia with a deficit of total body sodium. The patient with neither edema nor evidence of ECF volume depletion is classified as having hyponatremia with near-normal total body sodium (Fig. 2-1).
A. In the hypervolemic (edematous) hyponatremic patient, both total body sodium and TBW are increased, water more so than sodium. These patients have cardiac failure, cirrhosis, nephrotic syndrome, or renal failure. When hyponatremia is secondary to cardiac and hepatic disease, the disease is advanced and readily evident on clinical examination. In the absence of the use of diuretics, the urinary sodium concentration in the hyponatremic edematous patient should be quite low (<10 to 20 mEq/L) because of avid tubular sodium reabsorption. The exception occurs in the presence of acute or chronic renal failure, in which, because of tubular dysfunction, the urinary sodium concentration is higher (>20 mEq/L).
B. The diagnostic possibilities in the hypovolemic hyponatremic patient are entirely different. Again, a spot urinary sodium concentration is of value. If the volume-depleted hyponatremic patient has a low (<10 to 20 mEq/L)
urine sodium concentration, the kidney is functioning normally by conserving sodium in response to ECF volume depletion. This occurs with extrarenal fluid losses. Conversely, if the urinary sodium concentration is greater than 20 mEq/L in a hypovolemic hyponatremic patient, the kidney is not responding appropriately to the ECF volume depletion, and renal losses of sodium and water must be considered as the likely cause of the hyponatremia.
urine sodium concentration, the kidney is functioning normally by conserving sodium in response to ECF volume depletion. This occurs with extrarenal fluid losses. Conversely, if the urinary sodium concentration is greater than 20 mEq/L in a hypovolemic hyponatremic patient, the kidney is not responding appropriately to the ECF volume depletion, and renal losses of sodium and water must be considered as the likely cause of the hyponatremia.
1. In a hypovolemic hyponatremic patient with a urinary sodium concentration of less than 10 to 20 mEq/L, a gastrointestinal (or “third space”) source of sodium and water losses must be sought. The source may be readily apparent if the patient presents with a history of vomiting, diarrhea, or both. In the absence of an obvious history of gastrointestinal fluid losses, several other diagnostic possibilities must be considered. Substantial ECF losses may occur into the abdominal cavity with peritonitis or pancreatitis and into the bowel lumen with ileus or pseudomembranous colitis. The surreptitious cathartic abuser may present with evidence of ECF volume depletion and no history of gastrointestinal losses. The presence of hypokalemic metabolic acidosis and phenolphthalein in the urine may be a clue to this diagnosis. Loss of haustra on barium enema and melanosis coli on endoscopy are other clues to cathartic abuse. Burns or muscle damage may also lead to a state of hypovolemia and hyponatremia secondary to substantial fluid and electrolyte losses from skin or into muscle.
2. In a hypovolemic hyponatremic patient with a urinary sodium level of greater than 20 mEq/L, renal losses occur, and several different diagnostic possibilities must be considered.
a. Excessive use of diuretics is foremost among these diagnoses. It occurs almost exclusively with thiazide diuretics, because these agents,
unlike loop diuretics, alter only urinary diluting ability, and a urinary concentration remains unimpaired. A fall in plasma sodium concentration in a patient receiving diuretics may be the first clue that a diuretic dosage adjustment is needed. In some patients with diuretic abuse, ECF volume depletion is not readily apparent from clinical examination. An important clue, however, to the diagnosis of diuretic-induced hyponatremia is that virtually all these patients have an associated hypokalemic metabolic alkalosis if they are receiving potassium-losing diuretics. If, however, a potassium-sparing diuretic is involved (e.g., triamterene, amiloride, and spironolactone), neither hypokalemia nor metabolic alkalosis may be present. Cessation of use of the diuretic is the best means of confirming the diagnosis of diuretic-induced hyponatremia. However, it must be remembered that restoration of ECF volume is also necessary to correct the hyponatremia. This will improve renal function and suppress the hypovolemia-mediated nonosmotic release of vasopressin. In the hypokalemic patient, potassium replacement also may be necessary for complete correction of the plasma sodium concentration imbalance.
unlike loop diuretics, alter only urinary diluting ability, and a urinary concentration remains unimpaired. A fall in plasma sodium concentration in a patient receiving diuretics may be the first clue that a diuretic dosage adjustment is needed. In some patients with diuretic abuse, ECF volume depletion is not readily apparent from clinical examination. An important clue, however, to the diagnosis of diuretic-induced hyponatremia is that virtually all these patients have an associated hypokalemic metabolic alkalosis if they are receiving potassium-losing diuretics. If, however, a potassium-sparing diuretic is involved (e.g., triamterene, amiloride, and spironolactone), neither hypokalemia nor metabolic alkalosis may be present. Cessation of use of the diuretic is the best means of confirming the diagnosis of diuretic-induced hyponatremia. However, it must be remembered that restoration of ECF volume is also necessary to correct the hyponatremia. This will improve renal function and suppress the hypovolemia-mediated nonosmotic release of vasopressin. In the hypokalemic patient, potassium replacement also may be necessary for complete correction of the plasma sodium concentration imbalance.
Surreptitious diuretic abuse occurs among premenopausal women who use diuretics for weight loss or other cosmetic reasons (e.g., thick ankles or calves, “puffy” face). These patients may be difficult to distinguish from patients with surreptitious vomiting, because both may present with evidence of ECF volume depletion and hypokalemic metabolic alkalosis. The presence or absence of hyponatremia depends on the patient’s water intake. The pivotal diagnostic test to distinguish between the hypovolemic hyponatremic patient with metabolic alkalosis who is a diuretic abuser and the patient who is a surreptitious vomiter is the urinary chloride concentration. Surreptitious vomiters have low (<10 mEq/L) chloride concentrations and surreptitious diuretic abusers have high (>20 mEq/L) concentration.
b. Salt-Losing Nephritis. Patients with medullary cystic disease, chronic interstitial nephritis, polycystic kidney disease, analgesic nephropathy, partial urinary tract obstruction, and, rarely, chronic glomerulonephritis may present with hypovolemic hyponatremia secondary to salt-losing nephritis. These patients generally have moderately advanced renal impairment with serum creatinine levels greater than 3 to 4 mg/dL. This diagnosis should virtually never be considered in patients with renal disease that is not associated with elevated serum creatinine. Patients with salt-losing nephritis may need supplemental sodium chloride (NaCl) intake to avoid ECF volume depletion, or they may become very susceptible to ECF volume depletion in association with either decreased intake or extrarenal (e.g., gastrointestinal) sodium and water losses. Because these patients may be pigmented secondary to uremic dermatitis and exhibit hyponatremia and volume depletion, their disease was initially described as mimicking Addison disease.
c. Mineralocorticoid Deficiency. The patient with Addison disease (i.e., primary adrenal insufficiency) generally has associated hyperkalemia; prerenal azotemia most often does not increase serum
creatinine to concentrations greater than 3 mg/dL. In patients with mineralocorticoid deficiency, ECF volume repletion may correct both the hyponatremia and the hyperkalemia. During periods of stress, the plasma cortisol level may be within the normal range. Therefore, if adrenal insufficiency is suspected, a 2-hour cosyntropin (Cortrosyn) stimulation test should be performed. In addition to a urinary sodium concentration of greater than 20 mEq/L, a urinary potassium concentration of less than 20 mEq/L may be another clue to mineralocorticoid deficiency. If fluid intake has been restricted, the patient with Addison disease may not present with hyponatremia, and hyperkalemia may not be present if the ECF volume depletion is not severe. Therefore, a high index of suspicion is necessary to make the diagnosis of primary adrenal insufficiency. These patients may present with nonspecific symptoms such as weight loss, anorexia, abdominal pain, nausea, vomiting, diarrhea, and fever.
creatinine to concentrations greater than 3 mg/dL. In patients with mineralocorticoid deficiency, ECF volume repletion may correct both the hyponatremia and the hyperkalemia. During periods of stress, the plasma cortisol level may be within the normal range. Therefore, if adrenal insufficiency is suspected, a 2-hour cosyntropin (Cortrosyn) stimulation test should be performed. In addition to a urinary sodium concentration of greater than 20 mEq/L, a urinary potassium concentration of less than 20 mEq/L may be another clue to mineralocorticoid deficiency. If fluid intake has been restricted, the patient with Addison disease may not present with hyponatremia, and hyperkalemia may not be present if the ECF volume depletion is not severe. Therefore, a high index of suspicion is necessary to make the diagnosis of primary adrenal insufficiency. These patients may present with nonspecific symptoms such as weight loss, anorexia, abdominal pain, nausea, vomiting, diarrhea, and fever.
d. Osmotic diuresis obligating anion and cation excretion is another major diagnostic consideration in the hypovolemic hyponatremic patient with a urinary sodium concentration greater than 20 mEq/L.
i. Glucose, urea, or mannitol diuresis. The uncontrolled diabetic patient may have substantial glucosuria, causing water and electrolyte losses and thereby ECF volume depletion. The urea diuresis after the relief of a urinary tract obstruction is another example of an osmotic diuresis that can cause ECF volume depletion. A chronic mannitol infusion without electrolyte replacement can produce a similar situation.
ii. Bicarbonaturia. Increased anion excretion also can obligate renal water and electrolyte losses. The most frequently encountered example of this is metabolic alkalosis with bicarbonaturia. The bicarbonate anion in the urine is accompanied by cations, including sodium and potassium, which maintain electrical neutrality. Bicarbonaturia may accompany the early development of metabolic alkalosis accompanying postoperative nasogastric suction or vomiting. Proximal renal tubular acidosis (e.g., in Fanconi syndrome) is another condition in which bicarbonaturia causes renal electrolyte loss. In the absence of a urinary tract infection with urease-producing organisms, a urinary pH (measured by a pH meter) greater than 6.1 indicates the presence of bicarbonate in the urine.
iii. Ketonuria. Ketoacid anions also can obligate renal electrolyte losses in spite of ECF volume depletion; this may contribute to urinary electrolyte losses in diabetic or alcoholic ketoacidosis or starvation.
e. Cerebral salt wasting is a syndrome, described primarily in patients with subarachnoid bleeds, characterized by renal salt wasting leading to volume contraction and non-osmotic release of vasopressin. It is postulated that a brain hormone leads to the natriuresis. The diagnosis requires the presence of sodium in the urine in the face of substantive evidence for volume contraction. This criterion is rarely fulfilled, suggesting that the entity is quite rare and frequently overdiagnosed.
C. Euvolemic hyponatremia is the most commonly encountered form of hyponatremia in hospitalized patients. The urinary sodium concentration in euvolemic hyponatremia is generally greater than 20 mEq/L. However, if the patient is on a sodium-restricted diet or is volume depleted, the urinary sodium concentration may be less than 10 mEq/L. Refeeding with a normal salt intake or expansion of ECF volume with saline increases urinary sodium concentration to more than 20 mEq/L, but hyponatremia will persist in the patient with euvolemic hyponatremia. These patients show no signs of either an increase or decrease in total body sodium. Although the water retention leads to an excess in TBW, no edema is detected because two-thirds of the water is inside the cell. A limited number of diagnostic possibilities are available for hyponatremic patients who exhibit neither edema nor ECF volume depletion (i.e., euvolemic hyponatremic patients) (Fig. 2-1). Two endocrine disorders must be considered: severe hypothyroidism and secondary adrenal insufficiency associated with pituitary or hypothalamic disease.
1. The occurrence of hyponatremia with hypothyroidism generally suggests severe disease, including myxedema coma. In some patients, particularly the elderly, the diagnosis may not be readily apparent. Therefore, thyroid function must be assessed in the euvolemic hyponatremic patient.
2. Glucocorticoid Deficiency. An intact renin-angiotensin-aldosterone system avoids ECF volume depletion in patients with secondary adrenal insufficiency, but it is clear that glucocorticoid deficiency alone can impair water excretion and cause hyponatremia. Skull films and computed tomographic (CT) scans should always be obtained in the euvolemic hyponatremic patient when the cause of the hyponatremia is not obvious. However, normal skull films or CT scans do not exclude secondary adrenal insufficiency. A low plasma cortisol level associated with a low adrenocorticotropic hormone level supports the diagnosis of secondary adrenal insufficiency. In this setting, both secondary adrenal insufficiency and secondary hypothyroidism may contribute to the hyponatremia accompanying pituitary insufficiency.
3. Emotional or physical stress must be considered in the euvolemic hyponatremic patient before invoking the diagnosis of the syndrome of inappropriate antidiuretic hormone (SIADH). Acute pain or severe emotional stress (e.g., decompensated psychosis associated with continued water ingestion) may lead to acute and severe hyponatremia. It is likely that a combination of emotional stress and physical pain accounts for the frequently encountered secretion of vasopressin in the postoperative state, which in turn leads to hyponatremia in the face of hypotonic fluid administration.
4. A number of pharmacologic agents either stimulate the release of vasopressin or enhance its action. These include:
a. Nicotine
b. Chlorpropamide
c. Tolbutamide
d. Clofibrate
e. Cyclophosphamide
f. Morphine
g. Barbiturates
h. Vincristine
i. Carbamazepine (Tegretol)
j. Acetaminophen
k. Nonsteroidal anti-inflammatory drugs
l. Antipsychotics
m. Antidepressants
Therefore, determining whether the euvolemic hyponatremic patient is receiving such drugs is an important diagnostic step.
5. SIADH should be considered after exclusion of other diagnoses in the euvolemic hyponatremic patient. In general, the causes of SIADH include:
a. Carcinomas, most frequently but not exclusively, of the
i. Lung
ii. Duodenum
iii. Pancreas
iv. Head and neck
b. Pulmonary disorders, including but not limited to,
i. Viral pneumonia
ii. Bacterial pneumonia
iii. Pulmonary abscess
iv. Tuberculosis
v. Aspergillosis
c. Central Nervous System (CNS) Disorders
i. Encephalitis (viral or bacterial)
ii. Meningitis (viral, bacterial, or tubercular)
iii. Acute psychosis
iv. Stroke (cerebral thrombosis or hemorrhage)
v. Acute intermittent porphyria
vi. Brain tumor
vii. Brain abscess
viii. Subdural or subarachnoid hematoma or hemorrhage
ix. Guillain-Barré syndrome
x. Head trauma
d. Acquired Immunodeficiency Syndrome.
Therefore, SIADH occurs primarily in association with infections and with vascular and neoplastic processes in the CNS or lung.
e. Exercise-Induced Hyponatremia. Hyponatremia has been well described in association with strenuous exercise such as marathons. It appears that a BMI of <20 kg/m2 and prolonged running times are both risk factors. Most importantly, it has been noted that weight gain during the race is a strong risk factor. This gain is most likely a function of water consumption in excess of insensible losses in the presence of non-osmotic vasopressin release.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree