The Patient with Hematuria, Proteinuria, or Both, and Abnormal Findings on Urinary Microscopy
Godela M. Brosnahan
I. URINE ANALYSIS
A urine sample is usually easy to obtain, and it can provide important information in the evaluation of patients with hypertension (underlying kidney disease), edema (nephrotic syndrome), and elevated serum creatinine (acute kidney injury and/or chronic kidney disease). It is also useful in patients with symptoms such as dysuria, flank pain, or gross hematuria, because it may point to a specific diagnosis. However, it must always be interpreted in conjunction with the patient’s history, physical examination, and other laboratory findings. Correct interpretation requires practice and experience from the clinician. Urinalysis must be performed in patients with systemic diseases such as systemic lupus erythematosus (SLE) or vasculitis to detect asymptomatic kidney involvement early so that appropriate therapy can be instituted.
A complete urinalysis consists of gross inspection, dipstick evaluation, and microscopic examination by a trained clinician. Proper collection and prompt examination of the urine sample are essential for obtaining reliable results.
A. Method of Collection of Urine Specimens: Ambulatory patients usually are asked to provide a midstream urine sample after thorough cleaning of the external genitalia with moist wipes (see Table 8-1). Unless these procedures are followed, contamination of the urine with bacteria, squamous cells, and leukocytes from vagina, vulva, or foreskin is common and leads to misinterpretation.
In hospitalized patients who are unable to void, a catheter may be inserted to obtain a urine sample. If possible, at least 200 mL urine should pass through the catheter to flush out contaminating urethral contents before the specimen is collected.
In patients with indwelling urinary catheters, the sample should be obtained directly from the catheter tubing, to collect recently produced urine, as opposed to urine from the drainage bag which is often contaminated with debris.
Suprapubic aspiration is rarely performed if accurate evaluation for infection is required. A fine lumbar puncture needle with stylet in place is passed through sterilized suprapubic skin directly into a full bladder. Uncontaminated urine can then be aspirated.
Table 8-1. Guidelines for Collecting a Midstream Urine Sample | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
B. Gross Inspection: Normal urine is clear and light yellow. Urine that contains an abundance of cells or crystals may look turbid. The most common abnormal color is red to brown urine which is often due to blood (hematuria) but may have other causes. The first step in the evaluation is centrifugation of the urine to determine whether the red color is in the sediment or in the supernatant. If the sediment is red but not the supernatant, the patient has hematuria. If the supernatant is red, it should be tested for heme with a dipstick. If the supernatant is heme positive, the patient has either hemoglobinuria due to massive hemolysis or myoglobinuria due to rhabdomyolysis.
A red supernatant that is negative for heme can be due to medications (rifampin, phenytoin, phenazopyridine) or food dyes. Ingestion of beets or rhubarb and acute intermittent porphyria are also in the differential diagnosis.
White urine may be due to pyuria, chyluria, or propofol (often used as a sedative in the intensive care unit), whereas green urine can be seen after administration of methylene blue, propofol, or amitriptyline. Black urine can occur with hemoglobinuria or myoglobinuria. Urine that turns black after standing for some time is a sign of alkaptonuria (“black urine disease”), an inborn disorder of tyrosine metabolism; the black color is due to oxidation of urinary homogentisic acid. Purple urine can be due to bacteriuria in patients with urinary catheters (purple urine bag syndrome).
C. Dipstick Testing: Dipstick testing of urine provides a rapid determination of urine pH, specific gravity, and the presence of protein, blood (hemoglobin), leukocytes, nitrites, glucose, and bile. It is important that the sample is tested promptly, because urine pH may change with time after collection, and contaminating bacteria multiply, converting nitrate to nitrite and causing a false-positive test result for bacteriuria. For confirmation of infection, a urine culture is required (see Chapter 7).
Increasing concentrations of protein in the urine cause a color change of the dipstick indicator from yellow to deepening shades of green. Because this is dependent on the concentration of the urine, very dilute urine will
result in underestimation of the amount of proteinuria (excreted over 24 hours) and very concentrated urine leads to overestimation. Therefore, a positive dipstick test for protein needs to be followed by a quantitative determination of protein excretion (see below). False-positive dipstick results for proteinuria are seen when urine pH is 8 or greater and when the patient is excreting metabolites of penicillins, aspirin, or oral hypoglycemic agents. It is important to bear in mind that standard dipsticks detect mainly albumin, but not light chains or immunoglobulins. Therefore, patients with multiple myeloma may have a negative result for protein on dipstick testing but large amounts of protein in a 24-hour urine collection. The dipstick also cannot detect very small quantities of albumin (e.g., microalbuminuria, see below).
result in underestimation of the amount of proteinuria (excreted over 24 hours) and very concentrated urine leads to overestimation. Therefore, a positive dipstick test for protein needs to be followed by a quantitative determination of protein excretion (see below). False-positive dipstick results for proteinuria are seen when urine pH is 8 or greater and when the patient is excreting metabolites of penicillins, aspirin, or oral hypoglycemic agents. It is important to bear in mind that standard dipsticks detect mainly albumin, but not light chains or immunoglobulins. Therefore, patients with multiple myeloma may have a negative result for protein on dipstick testing but large amounts of protein in a 24-hour urine collection. The dipstick also cannot detect very small quantities of albumin (e.g., microalbuminuria, see below).
Dipstick testing is very sensitive for heme in the urine, but it cannot differentiate between hemoglobin in red blood cells (RBCs; hematuria) and free hemoglobin or myoglobin, which are present in urine from patients with intravascular hemolysis or rhabdomyolysis. Ascorbic acid, a strong reducing agent, prevents the chemical reaction in dipsticks that detects hemoglobin and may be a cause of false-negative test results in subjects who ingest large amounts of vitamin C. False-positive results are even more common and may be due to the presence of semen in the urine, to a very alkaline urine pH or contamination with oxidizing agents used to clean the perineum. Therefore, a positive dipstick result for heme does not equal a diagnosis of hematuria; this must be established by microscopic examination.
Dipsticks can also detect leukocyte esterase which is released by lysed neutrophils and macrophages in urine. A positive test is a surrogate for pyuria (white blood cells in the urine); however, there are several causes for false-positive (e.g., very dilute urine) and false-negative (e.g., concentrated urine or the presence of proteinuria and glucosuria) test results. Moreover, many conditions can cause pyuria, and therefore microscopic examination of the urine and urine culture are still required to confirm or rule out urinary tract infection.
D. Microscopic Analysis: As stated above, microscopic analysis of the urine is essential because dipstick testing alone can be misleading and cannot identify the presence of renal epithelial cells (a marker of acute kidney injury, see Chapter 10), casts, or crystals. In the United States, urine is usually examined using a standard light microscope, after 10 mL of urine is centrifuged at 400-450 g for 5 minutes. The supernatant is then poured out, the pellet is resuspended, and a small drop is placed on a glass slide and covered with a coverslip. The specimen is then examined at low power (10×) to look for casts and at high power (40×) to count the number of RBCs, white blood cells, and epithelial cells per high-power field. This yields a semiquantitative estimate of the frequency of these cells in the urine.
More quantitative measures can be obtained by examining the urine in a counting chamber rather than on a plain glass slide, but this is usually not available for routine use in the United States. Phase-contrast microscopy is more sensitive for the observation of morphologic detail such as dysmorphic RBCs and should be used when available.
Most large clinical laboratories now perform the microscopic examination of the urine with automated analyzers utilizing flow cytometry. These accurately quantify urine elements such as epithelial cells, RBCs,
and white blood cells, but a qualitative morphologic assessment is not performed. Therefore, a distinction between dysmorphic glomerular hematuria and eumorphic hematuria of lower tract origin (see below) still requires manual examination. In addition, cellular and noncellular casts, crystalluria, and budding yeast are still better evaluated manually than by automated analyzers.
and white blood cells, but a qualitative morphologic assessment is not performed. Therefore, a distinction between dysmorphic glomerular hematuria and eumorphic hematuria of lower tract origin (see below) still requires manual examination. In addition, cellular and noncellular casts, crystalluria, and budding yeast are still better evaluated manually than by automated analyzers.
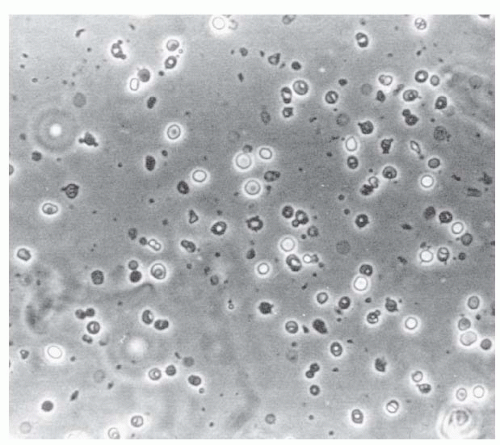
1. Hematuria: Abnormal hematuria is commonly defined as three or more RBCs per high-power field. Microscopic hematuria is only detected by examining the urine under the microscope, whereas macroscopic hematuria means visibly red to brown urine. Both can be due to glomerulonephritis or arise from an extraglomerular source, including the collecting system, ureter and bladder. Microscopic urinalysis may be useful in distinguishing between the two. Glomerular hematuria is likely if hematuria is accompanied by significant proteinuria, if the red cells have a dysmorphic appearance (i.e., red cells with blebs, budding, vesicle-shaped protrusions, and marked variability in shape and size, see Fig. 8-1), and if there are red cell casts (see below). In patients with macroscopic hematuria, a glomerular source is suggested
by a dark, cola-like color of the urine. This color arises from the formation of methemoglobin during prolonged passage of the red cells through the nephron in an acidic environment. If the urine is alkaline, glomerular bleeding may result in red urine. Nonglomerular bleeding is characterized by red to pink urine and microscopically by red cells that are round and uniform in size and shape, but there may be some “ghost cells,” that is, cells that are losing their hemoglobin (Fig. 8-2), which can occur in acid urine. Hematuria with passage of clots almost always arises from a lower urinary tract source (collecting system and/or bladder).
by a dark, cola-like color of the urine. This color arises from the formation of methemoglobin during prolonged passage of the red cells through the nephron in an acidic environment. If the urine is alkaline, glomerular bleeding may result in red urine. Nonglomerular bleeding is characterized by red to pink urine and microscopically by red cells that are round and uniform in size and shape, but there may be some “ghost cells,” that is, cells that are losing their hemoglobin (Fig. 8-2), which can occur in acid urine. Hematuria with passage of clots almost always arises from a lower urinary tract source (collecting system and/or bladder).
2. Urinary Leukocytes (Pyuria): Normal midstream urine contains up to 2,000 nucleated cells/mL, mostly leukocytes, whereas normal bladder urine obtained by needle aspiration contains very low numbers of leukocytes (mean, 283/mL). The higher counts in midstream urine are likely due to contamination from the urethra or in women from the vagina.
An increase in urinary leukocyte count (>20,000 leukocytes/mL or >5 leukocytes per high-power field) may be due to infection (see Chapter 7), but it also occurs in other conditions. When pyuria is present without bacteriuria, three-fourths of patients show an
underlying urinary tract abnormality such as acute or chronic interstitial nephritis, renal papillary necrosis and analgesic nephropathy, nephrolithiasis, glomerulonephritis, and polycystic kidney disease. In these conditions, the urine often contains red cells as well. If it is difficult to distinguish leukocytes from renal tubular epithelial cells, a drop of acetic acid makes it easier to recognize the lobed nuclei of polymorphonuclear leukocytes.
underlying urinary tract abnormality such as acute or chronic interstitial nephritis, renal papillary necrosis and analgesic nephropathy, nephrolithiasis, glomerulonephritis, and polycystic kidney disease. In these conditions, the urine often contains red cells as well. If it is difficult to distinguish leukocytes from renal tubular epithelial cells, a drop of acetic acid makes it easier to recognize the lobed nuclei of polymorphonuclear leukocytes.
3. Renal Tubular Cells: Large numbers of renal tubular cells in the urine can be found in acute tubular necrosis and acute interstitial nephritis. Acute interstitial nephritis can be distinguished from acute tubular necrosis if concomitant pyuria, microhematuria, and eosinophiluria are present. Eosinophils in the urine are best seen with Hansel’s stain, which, unlike Wright’s stain, is not pH dependent. However, acute interstitial nephritis can be present even in patients with few or no urinary abnormalities. Therefore, if clinical suspicion is high, renal biopsy should be performed.
Nucleated cells in the urine may also be found in patients with glomerulonephritis, particularly crescentic glomerulonephritis, in which red cells, white cells, and renal tubular cells are present in higher numbers than in noncrescentic glomerulonephritis. Glomerular epithelial cells (podocytes) may also appear in urine from patients with crescentic glomerulonephritis. These cells can be identified by monoclonal antibody staining for podocyte-specific proteins such as nephrin, but this is available only in research settings.
4. Urinary Casts: Casts are cylindrical structures that are formed in the tubular lumen from an organic matrix and may contain red or white blood cells, renal tubular cells, crystals, lipid, or bile. The major component of the matrix is Tamm-Horsfall glycoprotein, which is synthesized and secreted in the ascending limb of the loop of Henle and in distal convoluted tubules. Some casts are physiologic, that is, seen in the urine of healthy subjects. These are hyaline casts, which are transparent and consist of the protein matrix only, and occasional granular casts, which contain granules (likely cellular debris) embedded in the matrix. The number of hyaline and granular casts in the urine may be increased by fever, exercise, and volume depletion. A large number of muddy-brown granular casts are characteristic of acute tubular necrosis (see Chapter 10).
Pathologic casts are those that have tightly packed RBCs or white blood cells or renal tubular epithelial cells embedded in their matrix. The finding of cellular casts indicates an intrarenal origin of these cells. Red cell casts (Fig. 8-3) are most commonly seen in patients with glomerulonephritis, but may also occur with acute interstitial nephritis. “White cell casts can be seen in patients with pyelonephritis or with noninfectious interstitial nephritis, but sometimes also with proliferative glomerulonephritis. Renal tubular epithelial cell casts are indicative of acute tubular necrosis or acute interstitial nephritis.
In nephrotic syndrome, casts usually contain fat particles of varying sizes and some contain oval fat bodies (fatty casts). Oval fat bodies are tubular cells laden with fat droplets. Because the fat bodies consist of cholesterol esters, they can be easily identified under polarized light by
their “Maltese cross” birefringence (Figs. 8-4 and 8-5). If fat particles are too small to show crosses, they appear as a faint glow in polarized light.
their “Maltese cross” birefringence (Figs. 8-4 and 8-5). If fat particles are too small to show crosses, they appear as a faint glow in polarized light.
Broad casts form in dilated tubules with little flow and usually signify advanced chronic kidney disease.
5. Crystals: Although crystals of calcium oxalate and uric acid may be seen in normal urine samples, large, bizarre crystals of any type, including calcium oxalate and uric acid, usually signify increased urinary excretion of these substances and may indicate calculous disease. Cystine crystals are always abnormal and indicate cystinuria (see Chapter 6). Massive calcium oxalate crystalluria suggests ethylene glycol overdose.
 Figure 8-5. The particle in Figure 8-4, when viewed with polarized light, shows the classic “Maltese cross.” (From Fairley KF. Urinalysis. In: Schrier RW, Gottschalk CW, eds. Diseases of the kidney, 4th ed. Boston, MA: Little, Brown and Company, 1988. Reprinted with permission.) |
II. CAUSES OF HEMATURIA
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree