Laparoscopic surgery offers several significant advantages over open surgery, including more rapid recovery, lower wound complication rates, shorter hospital stay with its associated cost savings, and avoidance of large, chevron-type incisions typically required for open pancreatic surgery with their significant long-term morbidity. The acceptance and widespread utilization of laparoscopic techniques in pancreatic surgery, however, has had a slow evolution for several reasons, including substantial technical challenges, the risk of serious complications because of the proximity of the pancreas to major vascular structures, and the need for skills in both advanced laparoscopic surgery and expertise in open pancreatic surgery. Since the first reported case of laparoscopic distal pancreatectomy, case studies and series have been accumulating in the literature describing the laparoscopic treatment of pancreatic diseases such as pancreatic necrosis, chronic pancreatitis, and benign and malignant pancreatic tumors. Fig. 1 shows the different surgical procedures on the pancreas. In this review, the focus is on the results of the laparoscopic procedures that have gained more widespread acceptance, such as left-sided pancreatic resections, enucleation of pancreatic tumors, pancreatic debridement, and pancreaticoduodenectomy for pancreatic head lesions.
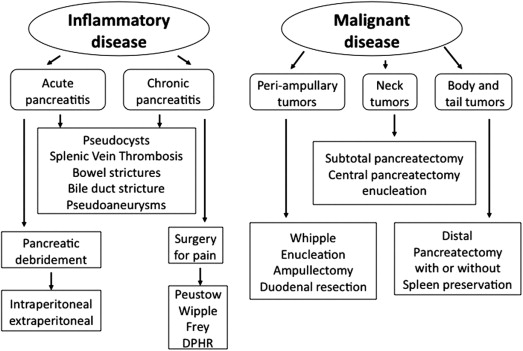
Surgical Techniques for Laparoscopic Surgery on the Pancreas
Different techniques have evolved for the minimal-access surgical approach to pancreatic surgery. In the pure laparoscopic approach, the surgical procedure is performed utilizing standard laparoscopic equipment and techniques. The advantage of this approach is that patients have only tiny incisions associated with the insertion of laparoscopic ports. The disadvantages of this approach include the absence of tactile sensation, the inability to retract tissues, and the increased difficulty in obtaining hemostasis if bleeding is encountered during surgery. A hand-assisted laparoscopic approach has evolved in which a specialized device, a handport, is utilized during laparoscopic surgery ( Fig. 2 ) . The handport allows for the maintenance of pneumoperitoneum and provides all the advantages of laparoscopic surgery, with the added benefit of having a hand in the abdomen. The disadvantage of this approach is that a surgical incision of about 2 to 4 inches is required to accommodate the surgeon’s hand. In the hybrid approach, the procedure is performed laparoscopically for mobilization and resection of the tumor; then, a small open incision is created, through which the procedure is completed. For example, this small open incision may be utilized for intestinal anastomosis during pancreaticoduodenectomy. It is unclear as to whether the hybrid approach provides any advantage over open surgery. Pure laparoscopic techniques and hand-assisted techniques are widely utilized for pancreatic surgery. Additionally, few reports have been published on the use of the DaVinci robot system for pancreatic surgery. At present, there is no evidence that the robotic approach provides any additional benefit over that of standard laparoscopic approaches.
Minimally Invasive Approach to the Treatment of Pancreatic Necrosis
Pancreatic necrosis, a complication of acute pancreatitis, is defined as a focal or diffuse area of nonviable pancreatic and peripancreatic tissue, and it can be associated with organ failure, hemorrhage, or infection. Sterile pancreatic necrosis is associated with a mortality rate of up to 12%. Infected pancreatic necrosis develops in up to 40% to 70% of cases and increases the mortality rate to as high as 30%. The traditional treatment for infected pancreatic necrosis has been open surgical debridement. The median mortality rate, however, of open surgical intervention is 25%, and ranges from 6% to 56%. A major development in the treatment of pancreatic necrosis has been the observation that areas of pancreatic necrosis that initially present as phlegmon or as diffuse necrosis undergo organization and localization—a process of forming a walled-off pancreatic necrosis (WON). This process usually takes at least 3 to 4 weeks after the initial insult to develop. Mortality rates drop sharply if operative intervention for pancreatic necrosis is delayed until WON has formed, because it facilitates complete necrosectomy ( Figs. 3 and 4 ). Present surgical practice strongly recommends against any intervention until there is evidence of WON on imaging studies. Minimally invasive techniques described to address pancreatic necrosis all require the presence of WON.
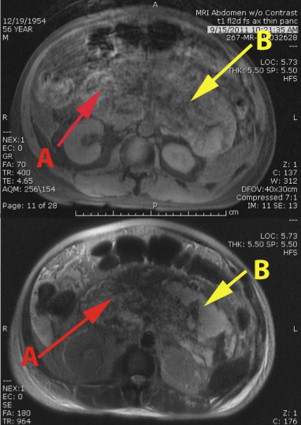
In treating a patient with necrotizing pancreatitis, it is important to distinguish a fluid collection from persistent pancreatic necrosis. Percutaneous drainage is useful for fluid collection owing to infected pseudocysts, but does not treat pancreatic necrosis in an area of WON or unorganized, diffuse, retroperitoneal pancreatic necrosis. Failure to recognize persistent pancreatic necrosis in WON can lead to significant delays in care and substantial morbidity. Magnetic resonance imaging, which is useful for detecting solid components of a collection, may prompt the treating physician to consider pancreatic debridement rather a simple percutaneous drainage of the WON or fluid collection, if there is debris within the collection (see Fig. 3 ).
The literature for minimally invasive techniques for pancreatic necrosis is limited to case studies and series. Percutaneous, endoscopic, retroperitoneal, and laparoscopic approaches have all been described. Percutaneous drainage is an important adjunct in the treatment of pancreatic necrosis because it assists in delaying operative intervention until WON has formed. It is also useful for taking care of residual collections formed after surgical debridement. Percutaneous drainage has also become an important component of the step-up approach for the treatment of sterile or infected WON. Horvath and colleageus reported on the efficacy of percutaneous drainage as part of the step-up approach in a multicenter, prospective, single arm, phase 2 study of video-assisted retroperitoneal debridement (VARD). Twenty-seven percent of patients in the study were treated primarily with percutaneous drainage and the authors found that a 75% reduction of the collection at 10 to 14 days after drainage was a predictor of successful percutaneous drainage alone. The Dutch group reported their results of a randomized, control study comparing the step-up approach to open necrosectomy for necrotizing pancreatitis. In this study of randomly assigned 88 patients, 35% of 44 patients randomized to the step-up approach responded to percutaneous drainage alone. These studies have suggested that patients requiring intervention for necrotizing pancreatitis should first have a percutaneous drain placed in the necrosis cavity. Patients who fail to respond within a short period of 1 to 2 weeks should then be crossed over to a surgical or an endoscopic intervention.
The use of percutaneous catheters as the definitive treatment of necrotizing pancreatitis has been reported in several small series of 20 to 30 patients, with success rates ranging from 30% to 100% of patients. Many patients treated with percutaneous catheters were hemodynamically stable and relatively healthy, thus making results difficult to extrapolate to patients with necrotizing pancreatitis, who are much more ill. Treatment using percutaneous catheters is very labor intensive, and requires multiple imaging studies with catheter placements, exchanges and debridement sessions. Gastrointestinal fistulas have been a significant complication of this method. Therefore, we do not recommend percutaneous drainage as the primary treatment for pancreatic necrosis.
Endoscopic debridement techniques that create an opening between the posterior gastric wall and the WON cavity have been described. In the majority of studies, an endoscopic ultrasound-guided approach is used. The endoscopic approach requires that the WON be optimally located and adherent to the stomach or duodenum. Patients with extension of necrosis into the paracolic gutters, and perinephric, retroduodenal, and other areas difficult to access through the endoscopic approach, or with large amounts of necrosis, are not suitable for endoscopic pancreatic debridement. A short metal stent is sometimes placed to maintain access to the WON cavity because multiple endoscopic procedures are usually required and the cavity is debrided using retrieval baskets and forceps. A successful debridement rate of 81% to 91% has been reported after median of 3 to 6 procedures per patient. Complications, which occurred in 15% to 26% of patients, included perforation, peritonitis, bleeding, and air embolism, requiring further treatment with angiographic or operative intervention. Endoscopic therapy requires highly specialized expertise found in a tertiary medical center. Additionally, multiple procedures performed nearly every other day may be required, which may be problematic in critically ill patients.
Minimally invasive surgical approaches that have been described include VARD and a laparoscopic intra-abdominal approach. In VARD, an endoscope or nephroscope is inserted after progressively dilating a drain tract or small incision so that a port can access the area of WON through a retroperitoneal approach. Once the WON cavity has been accessed, debridement is performed by piecemeal removal of the necrosis using a grasper inserted through the nephroscope. Multiple procedures are typically required for an adequate debridement. The benefits of this approach include avoiding opening the abdominal cavity, thereby potentially reducing the incidence of postdebridement sepsis with its associated systemic immune inflammatory syndrome–like response. The disadvantages of this approach are (i) the need for multiple procedures, (ii) the inability to address other intra-abdominal pathology such as gallstones, (iii) the inability to drain collections in areas, such as the periduodenal areas, transverse mesocolon, lesser sac, or root of the mesentery, which are not accessible through the retroperitoneum, and (iv) the possibility of inadequate debridement, because the small channel of the nephroscope provides limited access for debridement of large amounts of necrotic material.
Horvath and co-workers recently reported on a phase 2 single-arm multicenter study of VARD. Forty patients with infected pancreatic necrosis were evaluated with the step-up approach; of these, 31 patients required surgery. VARD was initially possible in 25 (81%) of surgical patients; however, 10 of these 25 patients (40%) ultimately required open surgery because of the technical inability to drain centrally located collections that were not accessible by VARD. Therefore, only 48% of the eligible 31 surgical patients were treated primarily by VARD, and in 51% of the surgical patients, open surgery was required to treat collections that were not accessible to VARD. Van Santvoort and associates reported on the results of a randomized, controlled trial comparing open necrosectomy with a step-up approach. Eighty-eight patients were randomized into either an open necrosectomy arm or a step-up arm. In the step-up arm 43 patients were initially treated with percutaneous drainage. Fifteen (35%) patients required percutaneous drainage only for the resolution of their necrosis. In the step-up arm, 26 patients were candidates for surgery owing to failure of percutaneous drainage alone. Twenty-four patients underwent VARD; in 14 of these patients (58%), further surgical procedures were necessary to address residual areas of necrosis or complications associated with VARD. Therefore, only 38% of patients requiring surgery in the step-up arm in this study were treated primarily by VARD. Patients who underwent the step-up approach with VARD had a lower rate of major complications or death and reduced utilization of health care resources compared with patients who underwent open necrosectomy. It is unclear whether these benefits can be attributed to VARD, because this study is fundamentally flawed. The step-up approach was utilized only in the VARD group (in 35% of patients in the step-up group, surgery was avoided with percutaneous drainage), whereas all patients in the open surgery arm underwent surgery. One plausible explanation for the poorer outcome for those in the open necrosectomy group is that those patients were not afforded the benefit of percutaneous drainage that those in the step-up arm received, allowing them to avoid surgery. It is possible that, if percutaneous drainage had been added as a treatment for the open necrosectomy group, a smaller number of patients may have required open necrosectomy with potentially better results. These multicenter studies demonstrate the limitations of VARD; only 40% to 50% of eligible patients were treated primarily by this approach because of the inaccessibility of centrally located areas of necrosis and the limited access for debridement of large amounts of necrotic material.
Gagner reported the first cases describing a laparoscopic approach to the treatment of necrotizing pancreatitis. Since that time, only a few case reports and small case series of patients undergoing laparoscopic pancreatic debridement have been reported ( Table 1 ). We have reported the largest experience of laparoscopic pancreatic necrosectomy, which initially included a series of 19 patients who underwent hand-assisted laparoscopic debridement of pancreatic necrosis as the initial treatment of necrotizing pancreatitis. One patient was converted to an open procedure secondary to an intraoperative enteric injury. Of the 18 patients who successfully underwent the initial laparoscopic pancreatic debridement, 4 patients required further surgical explorations: 2 of these were operated on during the early part of our experience and their subsequent procedures were performed by open surgery and the other 2 were reexplored laparoscopically. Thus, a total of 20 laparoscopic procedures were completed in 18 patients and 16 patients were treated by the laparoscopic route alone. There were 2 deaths in this series for a mortality rate of 11%. Mean length of stay was 16 days and external pancreatic fistulae developed in 11 (61%) patients.
| Author/Year | Patients (n) | Approach | Mortality | LOS (d) | Complications |
|---|---|---|---|---|---|
| Parekh/2010 | 49 | Transgastric, retrocolic, lesser sac, retroduodenal | 6% | NR | 35% Major complications |
| Bucher/2008 | 8 | Via drain tract | 0 | NR | 13% Pancreatic fistula |
| Parekh/2006 | 18 | Infracolic, lesser sac | 11% | 16 | 58% Pancreatic fistula |
| Zhou/2003 | 13 | Lesser sac, utz-guided | 0 | NR | 8% Pancreatic pseudocyst |
| Ammori/2002 | 1 | Transgastric | 0 | 14 | None |
| Zhu/2001 | 10 | Lesser sac | 10% | 10–30 | None |
| Hamad/2000 | 1 | Lesser sac | 0 | 7 | 100% Pancreatic fistula |
| Alverdy/2000 | 2 | Via drain tract | 0 | NR | 100% Pancreatic fistula |
| Cuschieri/2000 | 2 | Infracolic | 0 | 21–30 | None |
| Gagner/1996 | 7 | Transgastric, retrocolic retrogastric | 0 | 51 | None |
We have recently reported our updated experience on 56 consecutive patients treated surgically for pancreatic debridement from 2001 to 2010. Seven patients underwent open surgery and 49 patients underwent laparoscopic pancreatic debridement. The reasons for open surgery in the 7 patients were colon perforation (n = 2) and severe abdominal distension or adherence of bowel to the anterior abdominal wall that would have prevented an adequate pneumoperitoneum (n = 4); 1 patient, who was moribund, would not have tolerated a laparoscopic procedure. Of the 49 patients who were approached primarily with a hand-assisted laparoscopic procedure, the procedure was completed laparoscopically in 47 patients. Two patients were converted to open surgery (one because of an enteric injury and the other because of the patient’s severe portal hypertension, which made the laparoscopic procedure unsafe), for a conversion rate of 4%. The surgery duration was an average of 132 minutes, blood loss was 295 mL, and median blood transfusion was zero units. Twenty-two additional procedures were performed laparoscopically on this group of patients, including 15 cholecystectomies. Fifty-five percent of patients had an infected necrosis confirmed on intraoperative cultures. Twenty reoperations were performed in 21% of the patients for recurrent collections; 10 of these procedures were performed laparoscopically and 10 were open. The overall incidence of serious postoperative complications was 35% with the majority of the complications being non–life-threatening (Clavien grade 3 in 27%, grade 4 in 4.2%, and grade 5 in 4.2%). The mortality rate was 6%.
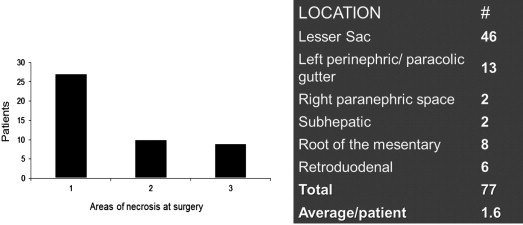
Endoscopic therapy and minimally invasive retroperitoneal approaches for pancreatic debridement require that the WON be located in an accessible location: The lesser sac adherent to the stomach for endoscopic approaches and the retroperitoneum for VARD. Both of these approaches are unsuitable when areas of necrosis are present in multiple compartments of the abdomen (see Fig. 3 ). Fig. 5 shows the 77 areas of necrosis found in the 47 patients in our study that underwent laparoscopic pancreatic debridement. There were 1.6 areas of necrosis per patient and all compartments of the abdomen were affected. Forty percent of patients had more than 1 area of necrosis in the abdomen. The wide distribution of the collections in different compartments of the abdomen suggests that many of these patients would have been unsuitable for minimally invasive endoscopic or VARD approaches. Utilizing the transabdominal laparoscopic approach, all identified areas of necrosis on the preoperative computed tomography (CT) were debrided at the initial procedure; we did not convert any patient to open surgery because of an inaccessible location or incomplete debridement of the pancreatic necrosis.
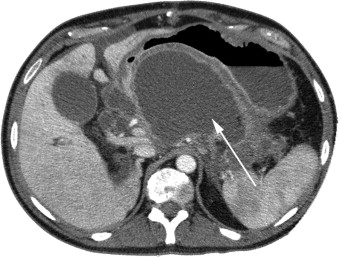
The transgastric approach for necrosectomy—opening the necrotic cavity into the stomach through the posterior gastric wall, which is similar to the endoscopic approach—avoids the creation of an external pancreatic fistula, providing a major advantage to these patients. We have thus far approached 2 patients laparoscopically with this technique of transgastric necrosectomy, with no morbidity or mortality (see Fig. 4 ). The laparoscopic transgastric approach provides an additional technique for debriding in selected patients with pancreatic necrosis.
Since 2001, we have laparoscopically debrided 67 patients with pancreatic necrosis successfully, with a mortality of 6% and a conversion rate of 3%. Our experience suggests that laparoscopic pancreatic debridement is an effective and safe procedure that allows for removal of both solid and liquid components of pancreatic necrosis in all intra-abdominal compartments with very low mortality and morbidity compared with open surgery. In patients with WON, a single procedure is usually required and conversion to open surgery is uncommon. It is a less traumatic way of achieving complete debridement of necrotic tissue in critically ill patients and may lead to a decreased systemic immune inflammatory syndrome–like response in the postoperative period, fewer complications, and a shorter hospital stay compared with open surgery. Other intra-abdominal pathology, such as gallstones, can be addressed simultaneously with the laparoscopic approach. Furthermore, our experience demonstrates that laparoscopic transgastric pancreatic necrosectomy may be preferred in selected clinically stable patients with a single area of lesser sac WON.
Laparoscopic pancreatic necrosectomy is a feasible option for the vast majority of patients requiring debridement for pancreatic necrosis. In our experience, the contraindications for the laparoscopic approach are few and are limited to (i) critically ill or moribund patients who will not tolerate pneumoperitoneum, (ii) patients with a markedly distended abdomen with adherence of bowel to the anterior abdominal wall making laparoscopic access to the abdominal cavity difficult, and (iii) patients who have other severe complications such as colonic necrosis, pseudoaneurysms, or severe left-sided portal hypertension from splenic vein or portal vein thrombosis.
Optimal treatment of necrotizing pancreatitis requires a multidisciplinary approach. Treatment procedures should be tailored to the patient based on the severity of the systemic illness, presence of infected necrosis, imaging localization of the WON areas, and the center’s expertise in minimally invasive techniques. Recent data suggest that the step-up approach may reduce morbidity and mortality compared with open necrosectomy. We agree with the recommendation that all patients requiring intervention for a sterile or infected necrosis should be treated with a percutaneous drain placed into the dominant collection. If there is a failure to respond rapidly, as demonstrated by improvement in the clinical condition and reduction of the cavity by about two thirds over a period of 1 to 2 weeks, then the patient should be crossed over to an endoscopic or surgical intervention.
Cumulative published data on minimally invasive approaches to pancreatic debridement suggest that it is a safe and effective option for all patients ranging from those who have an infected pseudocyst to those with large areas of walled off necrosis. Although VARD has been advocated as the preferred minimally invasive approach, recent studies show that only 40% to 50% of patients are suitable candidates for VARD as the primary approach. There are 2 major limitations of this approach: (i) Difficulty in accessing all the areas of necrosis in the abdomen and (ii) very limited access available through a nephroscope channel to remove large amounts of necrotic material. Our results suggest that the transabdominal laparoscopic approach may be preferred, because all the potential areas of necrosis in the abdominal cavity can be accessed through this approach. Open surgery for pancreatic debridement should be limited to the occasional patient who is unsuitable for minimally invasive approaches.
The specific minimally invasive approach selected depends on the expertise of the physician performing the procedure. Endoscopic and VARD approaches may be suitable for patients who have a small amount of necrosis and in whom the WON cavity is appropriately located, making the procedure technically feasible ( Table 2 ). Patients who have lesser sac collections that are adherent to the stomach may be treated through a transgastric approach by either an endoscopic or laparoscopic procedure (see Fig. 4 ). The laparoscopic procedure may be preferred if the physician has limited endoscopic experience and in patients who have large amounts of solid necrosis. Patients who are septic or critically ill and have a large or multiple areas of necrosis in different compartments of the abdomen on imaging should be considered primarily for the laparoscopic approach. The laparoscopic approach is presently underutilized; our experience suggests that it warrants a wider role in the treatment of necrotizing pancreatitis, because it is a technically feasible option in the vast majority of patients who require pancreatic debridement.
| n | LOS (d) | |
|---|---|---|
| Walled off largely liquid collection | 4 | 5 ± 2.7 |
| Walled off solid necrosis | 29 | 17.03 ± 2.36 |
| Partially walled off or diffuse solid necrosis | 6 | 78.8 ± 15.83 |
Minimally Invasive Approach to the Treatment of Pancreatic Necrosis
Pancreatic necrosis, a complication of acute pancreatitis, is defined as a focal or diffuse area of nonviable pancreatic and peripancreatic tissue, and it can be associated with organ failure, hemorrhage, or infection. Sterile pancreatic necrosis is associated with a mortality rate of up to 12%. Infected pancreatic necrosis develops in up to 40% to 70% of cases and increases the mortality rate to as high as 30%. The traditional treatment for infected pancreatic necrosis has been open surgical debridement. The median mortality rate, however, of open surgical intervention is 25%, and ranges from 6% to 56%. A major development in the treatment of pancreatic necrosis has been the observation that areas of pancreatic necrosis that initially present as phlegmon or as diffuse necrosis undergo organization and localization—a process of forming a walled-off pancreatic necrosis (WON). This process usually takes at least 3 to 4 weeks after the initial insult to develop. Mortality rates drop sharply if operative intervention for pancreatic necrosis is delayed until WON has formed, because it facilitates complete necrosectomy ( Figs. 3 and 4 ). Present surgical practice strongly recommends against any intervention until there is evidence of WON on imaging studies. Minimally invasive techniques described to address pancreatic necrosis all require the presence of WON.
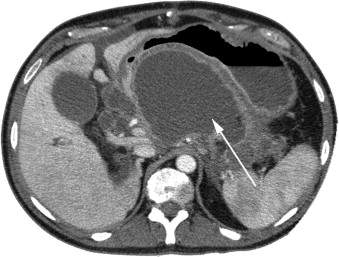
In treating a patient with necrotizing pancreatitis, it is important to distinguish a fluid collection from persistent pancreatic necrosis. Percutaneous drainage is useful for fluid collection owing to infected pseudocysts, but does not treat pancreatic necrosis in an area of WON or unorganized, diffuse, retroperitoneal pancreatic necrosis. Failure to recognize persistent pancreatic necrosis in WON can lead to significant delays in care and substantial morbidity. Magnetic resonance imaging, which is useful for detecting solid components of a collection, may prompt the treating physician to consider pancreatic debridement rather a simple percutaneous drainage of the WON or fluid collection, if there is debris within the collection (see Fig. 3 ).
The literature for minimally invasive techniques for pancreatic necrosis is limited to case studies and series. Percutaneous, endoscopic, retroperitoneal, and laparoscopic approaches have all been described. Percutaneous drainage is an important adjunct in the treatment of pancreatic necrosis because it assists in delaying operative intervention until WON has formed. It is also useful for taking care of residual collections formed after surgical debridement. Percutaneous drainage has also become an important component of the step-up approach for the treatment of sterile or infected WON. Horvath and colleageus reported on the efficacy of percutaneous drainage as part of the step-up approach in a multicenter, prospective, single arm, phase 2 study of video-assisted retroperitoneal debridement (VARD). Twenty-seven percent of patients in the study were treated primarily with percutaneous drainage and the authors found that a 75% reduction of the collection at 10 to 14 days after drainage was a predictor of successful percutaneous drainage alone. The Dutch group reported their results of a randomized, control study comparing the step-up approach to open necrosectomy for necrotizing pancreatitis. In this study of randomly assigned 88 patients, 35% of 44 patients randomized to the step-up approach responded to percutaneous drainage alone. These studies have suggested that patients requiring intervention for necrotizing pancreatitis should first have a percutaneous drain placed in the necrosis cavity. Patients who fail to respond within a short period of 1 to 2 weeks should then be crossed over to a surgical or an endoscopic intervention.
The use of percutaneous catheters as the definitive treatment of necrotizing pancreatitis has been reported in several small series of 20 to 30 patients, with success rates ranging from 30% to 100% of patients. Many patients treated with percutaneous catheters were hemodynamically stable and relatively healthy, thus making results difficult to extrapolate to patients with necrotizing pancreatitis, who are much more ill. Treatment using percutaneous catheters is very labor intensive, and requires multiple imaging studies with catheter placements, exchanges and debridement sessions. Gastrointestinal fistulas have been a significant complication of this method. Therefore, we do not recommend percutaneous drainage as the primary treatment for pancreatic necrosis.
Endoscopic debridement techniques that create an opening between the posterior gastric wall and the WON cavity have been described. In the majority of studies, an endoscopic ultrasound-guided approach is used. The endoscopic approach requires that the WON be optimally located and adherent to the stomach or duodenum. Patients with extension of necrosis into the paracolic gutters, and perinephric, retroduodenal, and other areas difficult to access through the endoscopic approach, or with large amounts of necrosis, are not suitable for endoscopic pancreatic debridement. A short metal stent is sometimes placed to maintain access to the WON cavity because multiple endoscopic procedures are usually required and the cavity is debrided using retrieval baskets and forceps. A successful debridement rate of 81% to 91% has been reported after median of 3 to 6 procedures per patient. Complications, which occurred in 15% to 26% of patients, included perforation, peritonitis, bleeding, and air embolism, requiring further treatment with angiographic or operative intervention. Endoscopic therapy requires highly specialized expertise found in a tertiary medical center. Additionally, multiple procedures performed nearly every other day may be required, which may be problematic in critically ill patients.
Minimally invasive surgical approaches that have been described include VARD and a laparoscopic intra-abdominal approach. In VARD, an endoscope or nephroscope is inserted after progressively dilating a drain tract or small incision so that a port can access the area of WON through a retroperitoneal approach. Once the WON cavity has been accessed, debridement is performed by piecemeal removal of the necrosis using a grasper inserted through the nephroscope. Multiple procedures are typically required for an adequate debridement. The benefits of this approach include avoiding opening the abdominal cavity, thereby potentially reducing the incidence of postdebridement sepsis with its associated systemic immune inflammatory syndrome–like response. The disadvantages of this approach are (i) the need for multiple procedures, (ii) the inability to address other intra-abdominal pathology such as gallstones, (iii) the inability to drain collections in areas, such as the periduodenal areas, transverse mesocolon, lesser sac, or root of the mesentery, which are not accessible through the retroperitoneum, and (iv) the possibility of inadequate debridement, because the small channel of the nephroscope provides limited access for debridement of large amounts of necrotic material.
Horvath and co-workers recently reported on a phase 2 single-arm multicenter study of VARD. Forty patients with infected pancreatic necrosis were evaluated with the step-up approach; of these, 31 patients required surgery. VARD was initially possible in 25 (81%) of surgical patients; however, 10 of these 25 patients (40%) ultimately required open surgery because of the technical inability to drain centrally located collections that were not accessible by VARD. Therefore, only 48% of the eligible 31 surgical patients were treated primarily by VARD, and in 51% of the surgical patients, open surgery was required to treat collections that were not accessible to VARD. Van Santvoort and associates reported on the results of a randomized, controlled trial comparing open necrosectomy with a step-up approach. Eighty-eight patients were randomized into either an open necrosectomy arm or a step-up arm. In the step-up arm 43 patients were initially treated with percutaneous drainage. Fifteen (35%) patients required percutaneous drainage only for the resolution of their necrosis. In the step-up arm, 26 patients were candidates for surgery owing to failure of percutaneous drainage alone. Twenty-four patients underwent VARD; in 14 of these patients (58%), further surgical procedures were necessary to address residual areas of necrosis or complications associated with VARD. Therefore, only 38% of patients requiring surgery in the step-up arm in this study were treated primarily by VARD. Patients who underwent the step-up approach with VARD had a lower rate of major complications or death and reduced utilization of health care resources compared with patients who underwent open necrosectomy. It is unclear whether these benefits can be attributed to VARD, because this study is fundamentally flawed. The step-up approach was utilized only in the VARD group (in 35% of patients in the step-up group, surgery was avoided with percutaneous drainage), whereas all patients in the open surgery arm underwent surgery. One plausible explanation for the poorer outcome for those in the open necrosectomy group is that those patients were not afforded the benefit of percutaneous drainage that those in the step-up arm received, allowing them to avoid surgery. It is possible that, if percutaneous drainage had been added as a treatment for the open necrosectomy group, a smaller number of patients may have required open necrosectomy with potentially better results. These multicenter studies demonstrate the limitations of VARD; only 40% to 50% of eligible patients were treated primarily by this approach because of the inaccessibility of centrally located areas of necrosis and the limited access for debridement of large amounts of necrotic material.
Gagner reported the first cases describing a laparoscopic approach to the treatment of necrotizing pancreatitis. Since that time, only a few case reports and small case series of patients undergoing laparoscopic pancreatic debridement have been reported ( Table 1 ). We have reported the largest experience of laparoscopic pancreatic necrosectomy, which initially included a series of 19 patients who underwent hand-assisted laparoscopic debridement of pancreatic necrosis as the initial treatment of necrotizing pancreatitis. One patient was converted to an open procedure secondary to an intraoperative enteric injury. Of the 18 patients who successfully underwent the initial laparoscopic pancreatic debridement, 4 patients required further surgical explorations: 2 of these were operated on during the early part of our experience and their subsequent procedures were performed by open surgery and the other 2 were reexplored laparoscopically. Thus, a total of 20 laparoscopic procedures were completed in 18 patients and 16 patients were treated by the laparoscopic route alone. There were 2 deaths in this series for a mortality rate of 11%. Mean length of stay was 16 days and external pancreatic fistulae developed in 11 (61%) patients.







