Chapter 20 The liver in heart failure
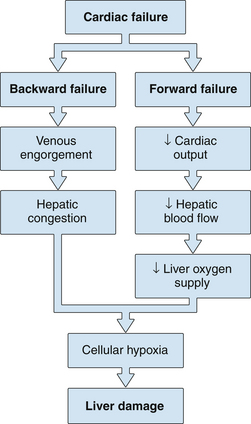
2 Backward heart failure causes congestion of the liver with hepatomegaly and nonspecific liver biochemical test abnormalities.
3 Forward heart failure causes ischemic damage to the liver if the circulatory failure is severe and prolonged. The pattern of a very rapid rise and fall in serum aminotransferase levels is characteristic.
4 Alternative diagnoses should be considered if abnormal liver biochemical test values are unusually high in the absence of an acute setting and, in particular, if the alkaline phosphatase level is more than twice normal or if the alanine aminotransferase (ALT) is much higher than the aspartate aminotransferase (AST) level.
5 The frequency and severity of liver involvement depend on the severity of heart failure, which determines prognosis unless cardiac cirrhosis is present.
6 No specific treatment is available for the liver dysfunction. Improvement in cardiac function results in return of liver biochemical test results to normal, unless cardiac cirrhosis is already present.
Overview
1. Liver dysfunction (cardiac hepatopathy) has long been recognized as a complication of both severe acute and chronic congestive heart failure.
2. Heart failure usually results from pump failure leading to decreased cardiac output, venous congestion, and retention of extracellular fluid.
Hepatic Circulation
Hepatic blood supply
1. The liver has a dual blood supply:
 The portal vein supplies approximately 66% to 83% of the blood flow to the liver and brings nutrient-rich but relatively less well-oxygenated venous blood from the stomach, intestine, and spleen.
The portal vein supplies approximately 66% to 83% of the blood flow to the liver and brings nutrient-rich but relatively less well-oxygenated venous blood from the stomach, intestine, and spleen.
 The portal vein supplies approximately 66% to 83% of the blood flow to the liver and brings nutrient-rich but relatively less well-oxygenated venous blood from the stomach, intestine, and spleen.
The portal vein supplies approximately 66% to 83% of the blood flow to the liver and brings nutrient-rich but relatively less well-oxygenated venous blood from the stomach, intestine, and spleen.2. A reduction in portal inflow or hepatic sinusoidal pressure results in a reflex increase in hepatic arterial blood flow and thereby ensures a constant sinusoidal pressure.
3. Primary changes in hepatic arterial blood flow are not associated with changes in portal venous blood flow.
4. A decrease in cardiac output usually results in reduced hepatic blood flow. The percentage of cardiac output received by the liver, however, remains relatively stable.
5. Decreased perfusion is usually compensated for by increased oxygen extraction, which can increase up to 95%.
Hepatic venous drainage
1. The liver is drained by the hepatic vein, which is formed by the right, middle, and left hepatic veins.
Hepatic microcirculation
1. The portal vein and the hepatic artery divide into branches to the right and left lobes of the liver. These branches further subdivide five to six times until their terminal branches reach the portal triads.
2. The portal vein tributaries open directly into hepatic sinusoids. The hepatic artery branches open into some but not all sinusoids. The sinusoids anastomose freely at all levels between the portal vein tributaries and the terminal hepatic venules.
3. Hepatic sinusoids have the following characteristics:
 They are lined by both endothelial cells and specialized macrophages called Kupffer cells. No basement membrane underlies the endothelial cells.
They are lined by both endothelial cells and specialized macrophages called Kupffer cells. No basement membrane underlies the endothelial cells.
 The porous nature of the sinusoids allows for low hydrostatic pressure and free flow between the sinusoids and the interstitial space, the space of Disse.
The porous nature of the sinusoids allows for low hydrostatic pressure and free flow between the sinusoids and the interstitial space, the space of Disse.
 They are lined by both endothelial cells and specialized macrophages called Kupffer cells. No basement membrane underlies the endothelial cells.
They are lined by both endothelial cells and specialized macrophages called Kupffer cells. No basement membrane underlies the endothelial cells. The porous nature of the sinusoids allows for low hydrostatic pressure and free flow between the sinusoids and the interstitial space, the space of Disse.
The porous nature of the sinusoids allows for low hydrostatic pressure and free flow between the sinusoids and the interstitial space, the space of Disse.Liver Architecture
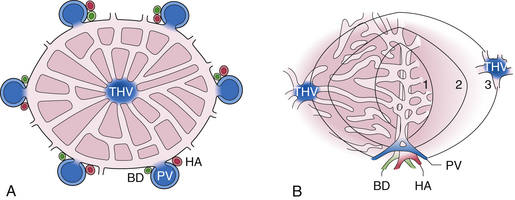
2. The functional unit of the liver is the acinus (Fig. 20.1B):
 Liver parenchymal cells are grouped into concentric zones centered around the portal triad; zone 1 is nearest, whereas zones 2 and 3 are more distal to the afferent blood vessels.
Liver parenchymal cells are grouped into concentric zones centered around the portal triad; zone 1 is nearest, whereas zones 2 and 3 are more distal to the afferent blood vessels.
 Liver parenchymal cells are grouped into concentric zones centered around the portal triad; zone 1 is nearest, whereas zones 2 and 3 are more distal to the afferent blood vessels.
Liver parenchymal cells are grouped into concentric zones centered around the portal triad; zone 1 is nearest, whereas zones 2 and 3 are more distal to the afferent blood vessels.Pathophysiology
4. Both forward failure and backward failure of the heart lead to cellular hypoxia and liver damage.
Chronic passive congestion
1. In heart failure with low cardiac output, total hepatic blood flow falls by approximately one third.
2. The increased systemic venous pressure is reflected as hepatic venous hypertension, which can cause hepatic cell atrophy as a result of sinusoidal congestion and expansion.
3. The accompanying perisinusoidal edema can result in decreased diffusion of oxygen and other metabolites to hepatocytes.
4. Collagenosis of the space of Disse from chronic congestion may play a minor role in impairing oxygen diffusion.
Decreased hepatic blood flow
1. Increased oxygen extraction by the liver in states of low hepatic blood flow ensures constant oxygen consumption within wide limits of hepatic blood flow. The liver therefore does not suffer adverse effects of hypoxia as a result of decreased hepatic blood flow under basal conditions.
2. A greater than 70% reduction in hepatic blood flow decreases oxygen uptake, galactose elimination capacity, and adenosine triphosphate concentrations and increases the lactate/pyruvate ratio (an index of tissue hypoxia).
3. Hepatic arterial vasoconstriction with intense selective splanchnic vasoconstriction in states of significant hypoperfusion and shock causes hypoxic damage to the liver.
4. Hypoxic damage characteristically occurs in the area adjacent to the terminal hepatic vein (zone 3 of the acinus), the area farthest away from the oxygen-carrying blood supply.
5. Insufficient concentrations of substrates, accumulation of metabolites, and release of cytokines secondary to an inflammatory response all contribute to hypoxic damage even in the presence of systemic circulatory support.
6. Loss of mitochondrial oxidative phosphorylation, as a result of hypoxia, leads to impaired membrane function, disrupted intracellular ion homeostasis, and reduced protein synthesis.
7. Reperfusion injury can aggravate hepatic injury through the generation of reactive oxygen species once ischemic hepatocytes are reexposed to oxygen.
Pathology
Macroscopic
2. Nodularity is inconspicuous, but if nodular regenerative hyperplasia (see later) or cardiac cirrhosis is present, nodules may be seen.
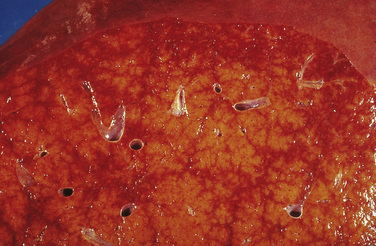
4. A “nutmeg” appearance results from the contrasting combination of hemorrhagic central areas of the lobules and the normal yellow portal and periportal areas (Fig. 20.4).
Microscopic
1. The severity of hepatic histopathologic changes generally correlates with the clinical or biochemical severity of heart failure and with cardiac weight and chamber size.
2. Early in heart failure, the terminal hepatic veins become engorged and dilated. Sinusoids adjacent to the terminal hepatic veins are also dilated and are filled with erythrocytes for a variable extent toward the portal areas. In severe cases, the appearance is that of peliosis hepatis (blood lakes).
3. Also evident are compression and variable atrophy of the liver cell plates and an apparent increase in the amount of lipofuscin in the cytoplasm of liver cells.
4. Moderately severe heart failure can result in zone 3 liver cell necrosis. The cellular infiltrate is inconspicuous.
6. The necrotic hepatocytes are often packed with a brownish pigment, which probably is related to bilirubin degradation.
7. Liver cell necrosis progresses from zone 3 to the portal areas as the heart disease progresses. In the most severe form of liver congestion from heart failure, only a small area of normal-appearing hepatocytes remains in the periportal area.
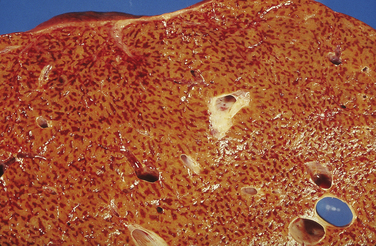
8. The reticulin network condenses and may collapse around the terminal hepatic vein following loss of liver cells (Fig. 20.5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree