Fig. 1
The appearance of CPP-containing sediments and contaminating debris following centrifugation of human serum. (a) Fibrin/red cell debris following centrifugation of serum for 30 min at 10,000 × g and 4 °C (i) and after TBS wash (ii). (b) CPP-containing pellets after centrifugation of supernatant in (a) for 2 h at 30,000 × g and 4 °C (i) and TBS wash (ii). As noted in text, pellets are small and translucent or slightly off-white in colouration and difficult to visualize. Arrows denote position of pellet
3.
Carefully transfer the supernatant to a clean safe-lock capped tube, taking care not to contaminate the supernatant with the pellet. If isolating CPP from urine do not discard the pellet at this stage.
For urine samples only:
4.
Deplete polymeric THP from urine by resuspending pellets from step 3 in 1 % (w/v) CHAPS in TBS and re-spinning for 30 min at 10,000 × g and 4 °C (see Note 8 ).
5.
Pool supernatants from steps 3 and 4 to maximize CPP recovery.
3.3.2 CPP Isolation by Centrifugation
1.
Mark the outer side of each safe-lock capped tube with a pen to indicate the orientation of the tube in the rotor and help locate the pellet (see Note 9 ).
2.
3.
Remove and discard as much supernatant as possible, then carefully wash and resuspend the pellet in 100–200 μL ice-cold TBS using a pipette. If the pellet is not visible, resuspend by flushing the pipette tip up and down the side of the tube where the pellet should be located (bottom of the tube on the side of the mark). Add ice-cold TBS to fill the tube.
4.
Centrifuge for 1 h at 30,000 × g and 4 °C and resuspend the pellet in 100 μL warmed (37 °C) TBS.
5.
Pool aliquots of the same sample and store at −80 °C until analysis.
3.3.3 CPP Isolation by Size Exclusion Chromatography (SEC)
1.
Apply 3 mL aliquots of diluted sample to a HiPrep 16/60 Sephacryl™ S-500 HR column equilibrated with calcium-supplemented TBS (Ca-TBS).
2.
Elute column isocratically with Ca-TBS at a flow rate of 0.5 mL/min with monitoring at 280 and 400 nm (Fig. 2).
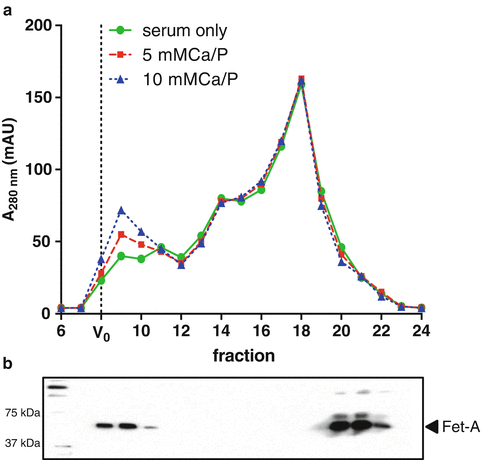

Fig. 2
CPP isolation by size exclusion chromatography. (a) Exemplar chromatographs showing the fractionation of fetuin-A -containing CPP from the fetuin-A monomer in human serum and serum spiked with buffered calcium and phosphate solutions (at the stated concentrations) monitored at 280 nm. CPP are eluted directly after the void volume (V 0). (b) Immunoblot of fetuin-A in eluted fractions
4.
Concentrate CPP-containing fraction, by passing eluate through a centrifugal filter unit (100 kDa MWCO). Collect the retentate.
5.
Pool as necessary and store at −80 °C until analysis.
3.3.4 CPP Immuno-Enrichment Using Magnetic Beads
Here, fetuin-A -containing CPP are selectively enriched from crude isolates by labeling particles with high-affinity biotinylated anti-Fetuin-A antibodies, which can then be captured using streptavidin-coated paramagnetic Dynabeads. Separation of this bound solid phase from the unbound phase is achieved with the use of a magnet.
1.
2.
Add 10 μg biotinylated anti-Fetuin-A antibody to the tube and mix.
4.
Mark the outer side of each safe-lock capped tube as described in step 1, Subheading 3.3.2. Centrifuge for 1 h at 30,000 × g and 4 °C to pellet CPP and separate from unbound antibody.
5.
Remove and discard as much supernatant as possible then carefully wash and resuspend the pellet in 200 μL ice-cold Isolation Buffer using a pipette. If the pellet is not visible, resuspend by flushing the pipette tip up and down the side of the tube where the pellet should be located (bottom of the tube on the side of the mark).
6.
Repeat spin steps 4 and 5, and resuspend pellet in 1 mL Isolation Buffer.
7.
Transfer 50 μL beads into a flat-bottomed tube and wash beads with 300 μL of Isolation Buffer. Mix well (see Note 14 ).
8.
Place the tube on the magnet for 1 min and discard the supernatant.
9.
Remove the tube from the magnet and add 1 mL of the antibody-bound CPP complex mixture. Mix well and incubate the tube for 20 h at 4 °C with mixing.
10.
Centrifuge the tube in a microfuge for 5 s to collect the sample at the bottom of the tube.
11.
Wash the bead-bound CPP by adding 500 μL of Isolation Buffer. Mix gently by pipetting up and down.
12.
Place the tube on the magnet for 1 min and discard the supernatant.
13.
Remove the tube from the magnet and repeat wash with 500 μL of Isolation Buffer. Mix gently by pipetting.
14.
Repeat steps 9 and 10 twice.
3.4 Confirming the Isolation of CPP
The techniques described in this section can be applied to bead-bound CPP isolates (Subheading 3.4.1). Conventional Western blotting and flow cytometry can be used to confirm the presence of protein markers ubiquitously present in CPP (e.g., fetuin-A , Apo-AI). The use of bead-bound CPP for staining for flow cytometry offers the advantage of be able to use a magnet to immobilize the bead for washing during separation of bound from unbound antibody, instead of more cumbersome and time-consuming centrifugation steps to pellet CPP after each wash step. It should be stressed that although Western blotting can be used to detect proteins present in purified CPP isolates, it does not confirm that they are necessarily of CPP origin and that contaminants are absent. Nonetheless, in our hands, markers of apoptotic bodies (e.g., calreticulin) and exosomes (e.g., CD63, CD81, CD9) are not usually detected in these preparations. Flow cytometry is more amenable to multiplexing targets and determining the relative abundance of various surface proteins. However, neither Western blotting nor flow cytometry of bead-bound CPP enable the user to discriminate between differences in the number of particles from changes in molecular composition/enrichment and therefore do not allow quantitation.
Analysis of particle size, morphology , crystallinity (e.g., diffraction studies), elemental composition (e.g., electron energy loss spectroscopy) and immunolabeling of free CPP can be made under cryo-TEM as reported previously (Fig. 3) [28]. An advantage of this EM modality is the avoidance of sample dehydration, collapse of the carbon (protein) matrix, and the use of chemical fixatives which can lead to artifactual appearances and compositional changes. Imaging studies can also help to assess sample heterogeneity and the presence of contaminants. Details of these EM methods are beyond the scope of this chapter and the interested reader is directed to other recent texts [29].
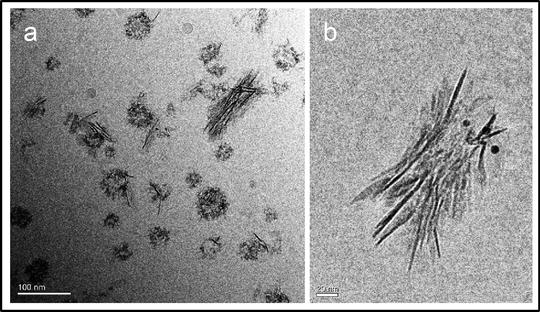

Fig. 3
Cryo-TEM analysis of fetuin-A -containing CPP isolated from human serum. (a) Micrograph showing heterogeneous population of mineral-containing nanoparticles isolated from human serum by differential centrifugation and immuno-enrichment (scale bar = 100 nm). (b) Immunogold labeling of fetuin-A in mature secondary CPP (scale bar = 20 nm)
3.4.1 SDS-PAGE/Western Blotting Analysis of CPP
1.
Place the tube containing bead-bound CPP on the magnet for 1 min and discard the supernatant (see Note 15 ).
2.
Add 25 μL 2× reducing Laemmli Sample Buffer (containing 40 mM EDTA) and vortex vigorously for 30 s.
3.
Incubate at 95 °C for 10 min.
4.
Place the tube in the magnet and load supernatant and molecular weight standards onto 4–15 % TGX gels. Run for ~45 min at 200 V.
5.
Transfer to 0.2 μm nitrocellulose using Trans-Blot Turbo (3 min, 2.5 A, 25 V).
6.
Block the membranes in BLOTTO for 2 h at room temperature.
7.
Incubate overnight at 2 °C (with gentle mixing) with primary anti-Fetuin-A or anti-ApoAI antibodies diluted 1 in 1000 in BLOTTO.
8.
Wash extensively in TBS-T (6 × 10 min).
9.
Incubate with species-matched TrueBlot HRP-conjugated secondary antibody (1 in 2000) plus StrepTactin-HRP conjugate (1 in 10,000) in BLOTTO for 2 h at room temperature.
10.
Wash extensively in TBS-T (6 × 10 min).
11.
Develop signal using SuperSignal West Dura Extended Duration Substrate and image after 5 min incubation at room temperature protected from light.
3.4.2 Staining CPP for Flow Cytometry
1.
Prepare staining antibody mix: combine 1 μL APOAI antibody (AF647), 1 μL Fetuin-A antibody (AF488) in 20 μL Isolation Buffer (see Note 16 ). This is sufficient for ten labeling reactions.









