A spectrum of oncologic treatments including chemotherapy, radiotherapy, and molecular targeted therapies is available to combat cancer. These treatments are associated with adverse effects in several organ systems including the gastrointestinal (GI) tract. The immunocompromised state induced by oncologic therapy is also an important contributing factor underlying GI complications. This review discusses common GI complications that can result from cancer therapy. The pathologic mechanisms underlying each complication and the pharmacology of the agents used to treat these complications are discussed.
A spectrum of oncologic treatments including chemotherapy, radiotherapy, and molecular targeted therapies is available to combat cancer. These treatments are associated with adverse effects in several organ systems including the gastrointestinal (GI) tract. Any part of the GI tract can be affected including the upper GI tract (esophagitis due to bacterial, viral, and fungal infections; mucositis due to chemotherapy or radiation; GI bleeding; nausea and vomiting), colon (diarrhea, graft-vs-host disease [GVHD], and constipation), liver (drug toxicity and GVHD), and pancreas (pancreatitis). Adverse effects range from mild to life threatening. The primary goal of cancer treatment is to administer the most effective therapy while minimizing potential toxicity. This review discusses common GI complications that can result from cancer therapy. The pathologic mechanisms underlying each complication and the pharmacology of the agents used to treat these complications are discussed.
Esophagitis
Esophagitis in patients with cancer may be caused either by the direct cytotoxic effects of chemotherapy or radiation or by the infections caused by immunosuppressive effects of cancer therapy ( Table 1 ). Treatment with chemotherapy or radiotherapy destroys rapidly dividing cells, such as those in the epithelial cell layer. Cell death decreases the renewal rate of the basal epithelium, causing mucosal atrophy, ulceration, and initiation of the inflammatory response. Synergy between chemotherapy and radiotherapy may increase the severity and extent of esophagitis observed with combined therapy. Esophagitis may also be caused by pill-induced injury, acid reflux disease, and GVHD in hematopoietic stem cell transplant recipients.
| Infectious Agent or Injury | Endoscopic Appearance | Treatment |
|---|---|---|
| Candida albicans | White plaquelike lesions with surrounding erythema on the esophageal mucosa | Systemic antifungal treatment with fluconazole, itraconazole, voriconazole, or echinocandins) |
| Herpes Simplex Virus | Small vesicles, coalescing to form ulcers | Acyclovir, foscarnet sodium |
| Cytomegalovirus | Linear or serpiginous ulcers | Ganciclovir, foscarnet sodium |
| Varicella-Zoster Virus | Small vesicles, similar to herpes simplex virus ulcers | Intravenous acyclovir |
| Polymicrobial Oral Flora | Bacteria mixed with necrotic epithelial cells in biopsy samples | Broad-spectrum antibiotics |
| Radiation Injury | Friable mucosa with erythema and edema | Lidocaine hydrochloride, proton pump inhibitors, endoscopic dilation, or stents |
Fungal Infections
Esophageal candidiasis is common in immunocompromised patients, with Candida albicans being the most frequent causative organism for esophageal and oropharyngeal candidiasis (OPC). Patients complain of odynophagia and/or dysphagia. On endoscopy, esophageal candidiasis is identified by white plaquelike lesions with surrounding erythema. Esophageal biopsies or brushings may confirm the presence of invasive yeast or hyphal forms of C albicans .
An empirical course of antifungal therapy is recommended in immunocompromised patients with odynophagia or dysphagia. Endoscopy should be performed if symptoms do not improve within 72 hours. The general duration of antifungal treatment is 14 to 21 days. Candida esophagitis in immunocompromised patients requires systemic antifungal therapy and cannot be treated with topical agents. Patients unable to tolerate oral agents require intravenous therapy.
The treatment of esophageal candidiasis includes agents such as azoles, echinocandins, or amphotericin B. Azoles inhibit cell membrane formation by inhibiting the synthesis of ergosterol, a principal component of fungal cell membranes. Fluconazole is the recommended first line agent because of its efficacy, ease of administration, and low cost. For patients with fluconazole-refractory esophageal candidiasis who can tolerate oral therapy, newer azoles (voriconazole and posaconazole) are available ( Table 2 ). Itraconazole has been found to be as effective as fluconazole for the treatment of esophageal candidiasis, however, its use is limited by significant nausea and the potential for drug interactions because of the inhibition of cytochrome P-450.
| First Line Therapy | Alternative Therapy | Comments | |
|---|---|---|---|
| Oropharyngeal Candidiasis | Clotrimazole troches; nystatin suspension or fluconazole | Itraconazole solution; or posaconazole or voriconazole or AmB-d oral suspension; IV echinocandin or AmB-d | Fluconazole is recommended for moderate to severe disease, and topical therapy with clotrimazole or nystatin is recommended for mild disease. Uncomplicated disease is treated for 7–14 d. For refractory disease, itraconazole, voriconazole, posaconazole, or AmB-d suspension is recommended |
| Esophageal Candidiasis | Fluconazole an echinocandin; or AmB-d | Itraconazole oral solution; or posaconazole or voriconazole | Oral fluconazole is preferred. For patients unable to tolerate an oral agent, IV fluconazole, an echinocandin, or AmB-d is appropriate. Treatment is for 14–21 d. For patients with refractory disease, the alternative therapy as listed or AmB-d or an echinocandin is recommended |
Patients requiring intravenous therapy should be treated with one of the echinocandins (caspofungin, micafungin, or anidulafungin), rather than amphotericin B, because of their better toxicity profiles. Echinocandins inhibit synthesis of β(1,3)- d -glucan, an essential component of the fungal cell wall. Mammalian cells do not require β(1,3)- d -glucan, thereby limiting potential toxicity. Relapse rates are higher with echinocandins when compared with azoles, and echinocandins are used as second line therapeutic agents if treatment with azoles has failed. Amphotericin B is reserved for esophageal candidiasis during pregnancy and in patients with drug-resistant candidiasis.
OPC is a local infection. Risk factors include radiation, chemotherapy, antibiotics, and corticosteroids. Treatment is with local agents such as nystatin or clotrimazole.
Prophylaxis
Patients at risk of developing OPC may be given antifungal prophylaxis with topical antifungals, such as clotrimazole or miconazole.
Viral Infections
Viral infections of the esophagus are caused by herpes simplex virus (HSV), cytomegalovirus (CMV), and uncommonly by, varicella-zoster virus (VZV). Diagnosis can be established by endoscopic biopsy. In advanced stages, all 3 viruses may cause small mucosal ulcerations. Biopsies taken from the edge of an HSV-related ulcer show intranuclear inclusions and multinucleated giant cells. Inclusions can also be detected by immunohistochemistry, using monoclonal antibodies to HSV. Biopsies of CMV lesions show intranuclear inclusions in fibroblasts and endothelial cells.
For patients with HSV esophagitis, acyclovir (400 mg orally 5 times daily for 14–21 days or 5 mg/kg intravenously every 8 hours for 7–14 days) is the therapeutic agent of choice. Acyclovir resistance in HSV results from mutations in the thymidine kinase (TK) gene of HSV. Viruses with TK mutations are generally cross-resistant to valacyclovir but remain susceptible to drugs that act directly on DNA polymerase, such as foscarnet. Cases of severe persistent infection with acyclovir-resistant HSV occur almost exclusively in immunocompromised patients. Famciclovir or valacyclovir can be considered in patients able to tolerate oral therapy, although there is limited clinical experience with these drugs in the treatment of HSV-associated esophagitis.
VZV esophagitis is initially treated with intravenous acyclovir because these patients usually have disseminated infection. After clinical improvement, treatment may be changed to an oral agent as used for HSV esophagitis.
CMV esophagitis is treated with intravenous ganciclovir (5 mg/kg twice daily) or foscarnet sodium (90 mg/kg twice daily) for 3 to 6 weeks. The role of maintenance treatment after the clearance of infection is not well defined. Valganciclovir is an oral precursor of ganciclovir. Although valganciclovir has been approved for treatment of CMV retinitis in patients with AIDS and is used for prophylaxis against CMV infection in solid-organ transplant recipients, its role in CMV GI disease has not been studied. At a dose of 900 mg daily, valganciclovir produces systemic drug exposure equivalent to 5 mg/kg of intravenous ganciclovir.
Bacterial Infections
Bacterial esophagitis can occur in immunocompromised patients and is usually polymicrobial, derived from oral flora. Diagnosis is made by endoscopic biopsies that demonstrate the presence of bacterial clusters mixed with necrotic epithelial cells. Treatment with broad-spectrum antibiotics is usually successful.
Radiation-Induced Esophagitis
Radiation-induced esophagitis can occur during external beam radiotherapy of lung, head and neck, and esophageal cancers. Acute radiation esophagitis is primarily caused by injury to the rapidly dividing cells of the basal epithelial layer, with subsequent thinning and denudation of esophageal mucosa. The severity of esophagitis depends on radiation dose and is exacerbated by concurrent use of chemotherapeutic agents such as cisplatin. Patients complain of odynophagia, dysphagia, and chest pain. Endoscopic findings include erythema, edema friable mucosa, ulcerations, or strictures.
Treatment includes use of local anesthetics such as viscous lidocaine hydrochloride and systemic narcotic analgesics and acid suppression with proton pump inhibitors and H 2 receptor antagonists. Esophageal strictures are treated by endoscopic dilation and refractory strictures may require placement of plastic stents. In patients with tracheoesophageal fistula due to esophageal cancer, self-expanding metal or plastic stents are the treatment of choice and they can achieve fistula closure in 70% to 100% of patients.
Diarrhea
Diarrhea is associated with several chemotherapeutic agents, particularly fluoropyrimidines such as 5-fluorouracil (5-FU) and capecitabine; irinotecan; and abdominal or pelvic radiotherapy. Other causes include small-molecule therapy, monoclonal antibodies, neutropenic enterocolitis, and Clostridium difficile infection (CDI).
Chemotherapy-Induced Diarrhea
Both 5-FU and irinotecan cause acute damage to intestinal mucosal epithelium leading to clinically significant diarrhea. The severity of chemotherapy-induced diarrhea is determined by the frequency and volume of stool output. Diarrhea is reported in up to 50% of patients receiving weekly 5-FU/leucovorin combined treatment. It tends to be worse in patients receiving irinotecan hydrochloride, 5-FU, and leucovorin than in those receiving 5-FU and leucovorin without irinotecan. Other factors that can increase the risk of 5-FU–induced diarrhea include female sex, the presence of an unresected primary tumor, and previous chemotherapy-induced diarrhea.
Irinotecan can cause early-onset diarrhea, which is mediated via cholinergic receptors, and can be effectively treated with atropine and loperamide hydrochloride. In contrast, late-onset diarrhea associated with irinotecan hydrochloride is unpredictable and occurs at all doses. It is seen less frequently when irinotecan is given every 3 weeks rather than weekly. Diarrhea also occurs frequently with regimens that combine 5-FU, leucovorin, and oxaliplatin.
Diarrhea commonly occurs in patients receiving small-molecule epidermal growth factor receptor–tyrosine kinase inhibitors. Grade 1 to 2 diarrhea, as defined by the National Cancer Institute’s common toxicity criteria, has been reported in up to 56% of patients receiving erlotinib. Another small-molecule inhibitor, sorafenib, is associated with diarrhea in approximately 34% of patients.
Radiation-Induced Diarrhea
Radiotherapy causes injury to the GI mucosa. Pelvic or abdominal radiotherapy can lead to acute enteritis, characterized by abdominal cramping and diarrhea in approximately 50% of patients. These symptoms are made worse by concomitant chemotherapy. Symptoms typically occur during the third week of fractionated radiotherapy.
Treatment of Chemotherapy- and Radiation-Induced Diarrhea
In 1998, Wadler and colleagues published guidelines on the treatment of chemotherapy-induced diarrhea. These guidelines were revised by an expert panel in 2004. The panel stressed the need for close monitoring of patients receiving a combination of irinotecan, 5-FU, and leucovorin and other intensive combination regimens, including weekly assessment of GI toxicity, particularly for older patients.
Opioid agonists are the cornerstone of therapy for chemotherapy-induced diarrhea. Loperamide and diphenoxylate are both widely used and are approved by the US Food and Drug Administration (FDA) for this indication; loperamide is more effective. For mild to moderate diarrhea, an initial dose of 4 mg of loperamide hydrochloride may be given, followed by a further 2-mg dose every 4 hours or after every stool discharge. Severe diarrhea often requires a more aggressive regimen, with an initial dose of 4 mg of loperamide hydrochloride followed by a further 2-mg dose every 2 hours or 4-mg dose every 4 hours until the patient is diarrhea-free for 12 hours.
This high-dose loperamide has been used effectively for the control of irinotecan-induced diarrhea. Octreotide, a synthetic long-acting somatostatin analogue, has been used as a second line therapeutic agent in opioid-resistant patients. It decreases the secretion of vasoactive intestinal peptide, prolongs intestinal transit time, and reduces secretion of intestinal fluid and electrolytes. The recommended initial dose of octreotide is 100 to 150 mcg given subcutaneously 3 times daily or 25 to 50 mcg/h every hour if given as an intravenous infusion. Sucralfate, a nonsystemic aluminum hydroxide complex, has been studied for control of radiotherapy-induced diarrhea and mucosal injury, with only limited, if any, benefit. In fact, sucralfate may aggravate GI symptoms such as rectal bleeding.
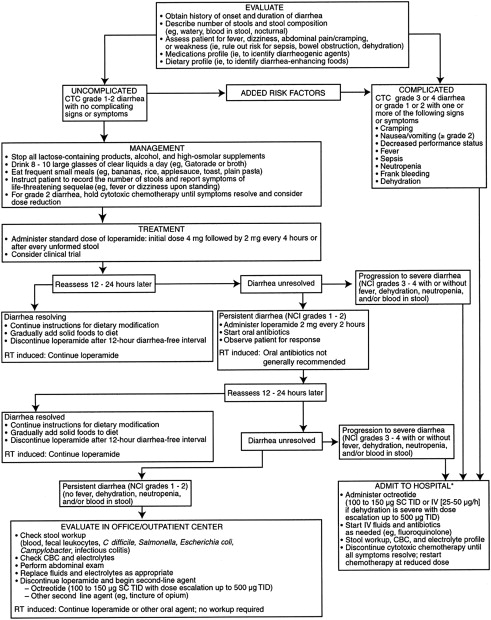
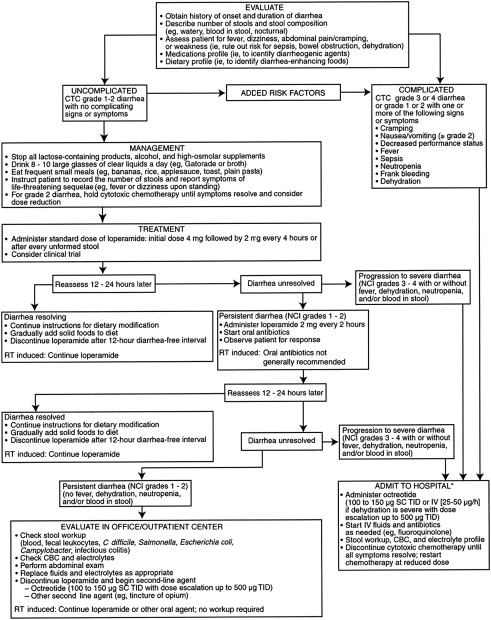
Other drugs used as adjunctive therapeutic agents in chemotherapy- or radiation-induced diarrhea include absorbents such as kaolin and charcoal, deodorized tincture of opium, paregoric, and codeine phosphate. Fig. 1 shows an algorithm for the management of chemotherapy-induced diarrhea.
Optimal dose of octreotide
Octreotide can be titrated to higher doses (500–2500 mcg 3 times daily) for the treatment of those who do not respond to lower doses. Early studies of octreotide for chemotherapy-induced diarrhea investigated subcutaneous doses ranging from 50 to 100 μg twice or thrice daily. Recent data suggest that higher doses may be more effective. Goumas and colleagues compared 100-μg octreotide with 500 μg administered 3 times a day in 59 patients with grade 3 or higher grade of chemotherapy-induced diarrhea who failed to respond to loperamide (4 mg 3 times a day) for at least 48 hours. Treatment with 500-μg octreotide was significantly more effective than with 100 μg (90% vs 61% of patients had complete resolution of diarrhea; P <.05), and both doses were well tolerated, suggesting that 500-μg octreotide given 3 times a day may be more effective than lower doses in patients who fail to respond to loperamide.
Role of prophylactic antidiarrheal therapy
Because of the well-recognized risk of diarrhea associated with irinotecan, recent studies have investigated prophylactic regimens for chemotherapy-induced diarrhea. Long-acting slow-release formulations of octreotide long acting release (octreotide LAR) can be administered by an intramuscular injection once a month. Once steady state has been achieved, administration of a 20-mg intramuscular dose of octreotide LAR every 4 weeks produces the same pharmacologic effects as 150-μg octreotide given thrice a day by subcutaneous injection and dramatically reduces fluctuations in peak and trough octreotide concentrations. Octreotide LAR, at a starting dose of 20 mg, effectively controls diarrhea associated with carcinoid syndrome, and monthly doses of 20 to 30 mg of octreotide LAR are currently being investigated for the treatment and prevention of chemotherapy-induced diarrhea.
Stem Cell Transplantation–Associated Diarrhea
Patients undergoing stem cell transplantation (SCT) can suffer from diarrhea caused by the conditioning regimen consisting of high-dose chemotherapy or radiotherapy, GVHD of the GI tract, or infection related to immunosuppressive therapy. Pretransplant conditioning regimens (including total body irradiation and/or combination chemotherapy) can injure the intestinal mucosa, as discussed earlier, causing secretory diarrhea that resolves after mucosal restitution. Transplant recipients of allogeneic stem cells can also develop GI GVHD, which usually starts 3 weeks or later after transplant, after engraftment of donor hematopoietic stem cells. GVHD and its associated diarrhea are discussed in a separate section later.
CDI
CDI is the most common nosocomial infection of the GI tract. Risk factors for CDI include a history of antibiotic therapy, bowel surgery, an immunocompromised state, and any process that suppresses the normal GI flora, including chemotherapy. CDI can occur up to 8 weeks after the end of a course of antibiotics, but patients undergoing cancer chemotherapy are predisposed to C difficile -induced diarrhea even in the absence of antibiotic therapy. Clinical presentation of CDI can vary from mild diarrhea to pseudomembranous colitis with or without protein-losing enteropathy to fulminant colitis with toxic megacolon.
A diagnosis is established by detecting C difficile toxin in stool or by identifying pseudomembranous colitis on endoscopic examination. Rapid enzyme immunoassays for detecting toxin A or B or both are now commonly used. Endoscopically, pseudomembranes can be seen as adherent yellow plaques that vary in diameter from 2 to 10 mm ( Fig. 2 ). The rectum and sigmoid colon are typically involved, but in approximately 10% of cases, colitis is only present in the more proximal colon and can be missed during sigmoidoscopy.
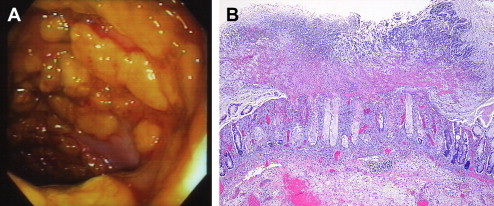
Standard therapy for C difficile -associated diarrhea is with oral metronidazole or oral vancomycin. Metronidazole at a dose of 500 mg 3 times daily or 250 mg 4 times a day given either orally or intravenously for 10 to 14 days is as effective as oral vancomycin given at a dose of 125 mg 4 times daily. The lower dose of vancomycin, 125 mg 4 times a day, is as effective as the higher dose, 250 mg 4 times a day, in case of mild to moderate diarrhea and is much less expensive.
Metronidazole has some advantages over vancomycin including lower cost and the observation that it can reduce selection of vancomycin-resistant enterococci. Metronidazole is, therefore, the initial therapy of choice in nonsevere cases of C difficile -induced diarrhea. If there is no improvement in 3 days, treatment with vancomycin should be initiated.
In patients with severe CDI and signs of systemic toxicity, the recommended treatment is vancomycin 125 mg orally 4 times daily, with dose escalation at 48-hour intervals up to 500 mg 4 times daily if patients fail to improve. If patients do not respond to oral vancomycin, the addition of intravenous metronidazole 500 mg every 8 hours or vancomycin retention enemas (0.5–1 g vancomycin dissolved in 1–2 L of normal saline every 4–12 hours) should be considered.
The use of antidiarrheal agents is not recommended because the decreased transit time can lead to complications and lengthen the duration of illness.
Relapse of CDI is common, occurring in up to 10% to 25% of all patients with CDI. Relapses usually occur within 1 to 3 weeks after ending initial therapy and are probably caused by failure to eradicate the organism rather than by antibiotic resistance. These patients are likely to relapse repeatedly. First relapses should be treated with a second 10- to 14-day course of oral metronidazole or vancomycin. If a patient relapses after taking a second course of antibiotics, different approaches have been suggested including tapered or pulsed antibiotic therapy, longer duration of treatment (several weeks), and the use of toxin-binding resins such as cholestyramine or colestipol hydrochloride alone or in combination with vancomycin. In a small series, 2 weeks of vancomycin administration followed by 2 weeks of rifaximin administration has proved successful in controlling recurrent disease. A recent study used 2 neutralizing human monoclonal antibodies against C difficile toxins A and B (CDA1 and CDB1, respectively) in 101 symptomatic patients, who were receiving either metronidazole or vancomycin. The rate of recurrence was significantly lower among patients treated with the monoclonal antibodies.
Diarrhea
Diarrhea is associated with several chemotherapeutic agents, particularly fluoropyrimidines such as 5-fluorouracil (5-FU) and capecitabine; irinotecan; and abdominal or pelvic radiotherapy. Other causes include small-molecule therapy, monoclonal antibodies, neutropenic enterocolitis, and Clostridium difficile infection (CDI).
Chemotherapy-Induced Diarrhea
Both 5-FU and irinotecan cause acute damage to intestinal mucosal epithelium leading to clinically significant diarrhea. The severity of chemotherapy-induced diarrhea is determined by the frequency and volume of stool output. Diarrhea is reported in up to 50% of patients receiving weekly 5-FU/leucovorin combined treatment. It tends to be worse in patients receiving irinotecan hydrochloride, 5-FU, and leucovorin than in those receiving 5-FU and leucovorin without irinotecan. Other factors that can increase the risk of 5-FU–induced diarrhea include female sex, the presence of an unresected primary tumor, and previous chemotherapy-induced diarrhea.
Irinotecan can cause early-onset diarrhea, which is mediated via cholinergic receptors, and can be effectively treated with atropine and loperamide hydrochloride. In contrast, late-onset diarrhea associated with irinotecan hydrochloride is unpredictable and occurs at all doses. It is seen less frequently when irinotecan is given every 3 weeks rather than weekly. Diarrhea also occurs frequently with regimens that combine 5-FU, leucovorin, and oxaliplatin.
Diarrhea commonly occurs in patients receiving small-molecule epidermal growth factor receptor–tyrosine kinase inhibitors. Grade 1 to 2 diarrhea, as defined by the National Cancer Institute’s common toxicity criteria, has been reported in up to 56% of patients receiving erlotinib. Another small-molecule inhibitor, sorafenib, is associated with diarrhea in approximately 34% of patients.
Radiation-Induced Diarrhea
Radiotherapy causes injury to the GI mucosa. Pelvic or abdominal radiotherapy can lead to acute enteritis, characterized by abdominal cramping and diarrhea in approximately 50% of patients. These symptoms are made worse by concomitant chemotherapy. Symptoms typically occur during the third week of fractionated radiotherapy.
Treatment of Chemotherapy- and Radiation-Induced Diarrhea
In 1998, Wadler and colleagues published guidelines on the treatment of chemotherapy-induced diarrhea. These guidelines were revised by an expert panel in 2004. The panel stressed the need for close monitoring of patients receiving a combination of irinotecan, 5-FU, and leucovorin and other intensive combination regimens, including weekly assessment of GI toxicity, particularly for older patients.
Opioid agonists are the cornerstone of therapy for chemotherapy-induced diarrhea. Loperamide and diphenoxylate are both widely used and are approved by the US Food and Drug Administration (FDA) for this indication; loperamide is more effective. For mild to moderate diarrhea, an initial dose of 4 mg of loperamide hydrochloride may be given, followed by a further 2-mg dose every 4 hours or after every stool discharge. Severe diarrhea often requires a more aggressive regimen, with an initial dose of 4 mg of loperamide hydrochloride followed by a further 2-mg dose every 2 hours or 4-mg dose every 4 hours until the patient is diarrhea-free for 12 hours.
This high-dose loperamide has been used effectively for the control of irinotecan-induced diarrhea. Octreotide, a synthetic long-acting somatostatin analogue, has been used as a second line therapeutic agent in opioid-resistant patients. It decreases the secretion of vasoactive intestinal peptide, prolongs intestinal transit time, and reduces secretion of intestinal fluid and electrolytes. The recommended initial dose of octreotide is 100 to 150 mcg given subcutaneously 3 times daily or 25 to 50 mcg/h every hour if given as an intravenous infusion. Sucralfate, a nonsystemic aluminum hydroxide complex, has been studied for control of radiotherapy-induced diarrhea and mucosal injury, with only limited, if any, benefit. In fact, sucralfate may aggravate GI symptoms such as rectal bleeding.
Other drugs used as adjunctive therapeutic agents in chemotherapy- or radiation-induced diarrhea include absorbents such as kaolin and charcoal, deodorized tincture of opium, paregoric, and codeine phosphate. Fig. 1 shows an algorithm for the management of chemotherapy-induced diarrhea.