Fig. 2.1
PillCam small bowel 3 capsule endoscope system
Table 2.1
Indication s for the use of capsule endoscopy according to anatomic site
Esophagus |
Gastroesophageal reflux disease |
Barrett’s esophagus |
Esophageal varices |
Small Bowel |
Obscure gastrointestinal bleeding |
Suspected Crohn’s disease |
Suspected small bowel tumor |
Evaluation of any abnormal small bowel imaging |
Evaluation of partially responsive celiac disease |
Surveillance of inherited polyposis syndromes |
Evaluation of drug-induced small bowel injury |
Evaluation of mucosal response to medications |
Colon |
Polyp screening |

Fig. 2.2
Various systems of capsule endoscopes available for the small bowel

Fig. 2.3
The Agile patency capsule system
The PillCam SB3 video capsule endoscopy system consists of (a) a 2 × 11 mm capsule containing the video camera, illumination, and batteries; (b) a sensing system comprising an array of sensor pads, a data recorder, and a battery pack; and (c) a workstation, based on a commercially available personal computer (Fig. 2.1). The new data recorders (DR3) also contain a portable real-time viewer that allows direct monitoring of the images received during the examination. While the PillCam captures images using a complementary metal-oxide semiconductor (CMOS) sensor, the EndoCapsule , MiroCam , and OMOM capsule use a charge-coupled device camera (CCD ). The four capsules also differ with regard to dimensions, image acquisition frame rate, field of view, and recording duration.
Almost all of the information provided in the literature is regarding the Given Imaging PillCam SB, as it dominated the market for a few years by itself, later on joined by the other small bowel capsules, and thus is the one on which most of the literature is written.
2.1.2 Small Bowel Video Capsule Endoscopy
Until the introduction of the small bowel video capsule endoscopy (SBCE ), the small bowel was an organ that was very difficult to explore with the available techniques. Since its development , SBCE provided a reliable, noninvasive, and well-accepted and well-tolerated procedure, which has revolutionized the study of the small bowel.
PillCam SB3 video capsule endoscope is a wireless capsule (11 × 26 mm) comprised of a light source, lens, CMOS imager, battery, and a wireless transmitter. A slippery coating allows easy ingestion and prevents adhesion of bowel contents, as it moves via peristalsis from the mouth to the anus (Figs. 2.1, 2.4). The battery provides >11 h of work in which the capsule photographs using an adaptive frame rate technique two to six images per second (>80,000 images all together), in a 156° field of view and 8:1 magnification. The pictures are transmitted via a newly developed ‘no attachments’ sensor belt, to a small data recorder (DR3) which also allows real-time imaging. The recorder is downloaded into a Reporting and Processing of Images and Data computer workstation (RAPID 8) and seen as a continuous video film. Support systems have been added since the first prototype of the RAPID system, including a localization system, a blood detector, a double and quadric picture viewer, a ‘quick viewer,’ single picture adjustment mode, incorporation of the Fuji Intelligent Color Enhancement (FICE) system, an inflammation (Lewis) scoring system, and an atlas, all meant to assist the interpreter.
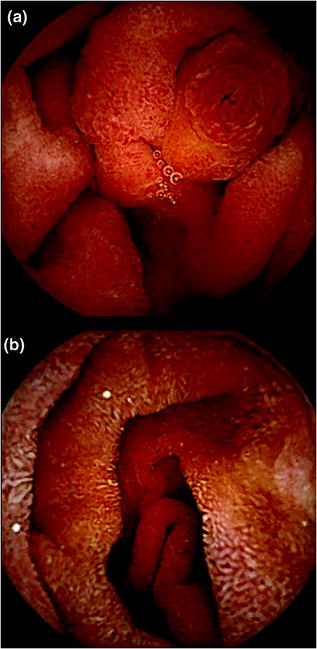

Fig. 2.4
Small bowel pictures taken with PillCam SB3: a Ampulla of Vater. b Small bowel normal mucosa
The procedure: The patient is on clear liquids the day prior to the procedure and swallows the capsule with water after a 12-h fast. Drinking clear fluids is allowed 2 h after ingestion as is a light lunch after 4 h. During the procedure, he is free to do his daily activities.
A few grading scales have been developed to assess the quality of bowel preparation in video capsule endoscopy , the most recent being a computer-assisted cleansing score (CAC) [7]. The impact of bowel preparation on the image quality and transit time was assessed in two meta-analyses. Preparation was found to improve the quality of visualization, but had no effect on transit times or percentage of capsules reaching the cecum, and no consensus was reached as to the effects on the diagnostic yield of the study [8, 9]. Another attempt to improve the small bowel diagnostic yield was attempted by using a capsule with two cameras (one on each side), which resulted in diagnosis of more lesions [10].
The main indication s for SBCE include the following:
1.
Obscure gastrointestinal bleeding
2.
Crohn’s disease (suspected/known)
3.
Suspected small bowel tumor
4.
Evaluation of abnormal small bowel imaging
5.
Partially/non-responsive celiac disease
6.
Surveillance of inherited polyposis syndromes
7.
Evaluation of drug-induced small bowel injury and response to medications
Contraindication s include the following:
1.
History of or suspected small bowel obstruction
2.
Swallowing disorders
3.
Pregnancy
4.
Non-compliance
Relative contraindication s are as follows:
1.
Major abdominal surgery in the previous 6 months.
2.
Cardiac devices—pacemaker/defibrillator.
Although the capsule is easily ingested and swallowed by most individuals, patients with severe dysphagia , large Zenker’s diverticulum, pill phobia, significant gastroparesis , and small children may have problems ingesting the device. For these situations, a capsule-loading device (AdvanCE, US Endoscopy, Mentor, Ohio, USA) is available to directly deliver the capsule into the stomach or duodenum.
In case of suspected small bowel obstruction , the use of a patency capsule (the AGILE capsule, Given Imaging , Yokneam, Israel) has been shown to provide evidence of the functional patency of the gastrointestinal tract [10] (Fig. 2.3). This system consists of a self-disintegrating capsule without a camera that contains radio frequency identification (RFID) tag and a RFID scanner. In a case of obstructive small bowel pathology, the AGILE capsule disintegrates within 30 h, and the remnants can pass through even small orifices [11]. The radio-opaque capsule can be detected by plain abdominal X-ray.
2.1.3 Occult GI Bleeding
Occult GI bleeding accounts for up to two-thirds of SBCE studies performed [12]. It was shown that 20–38 % of patients with normal upper and lower endoscopy have significant intestinal lesions [13, 14] (Fig. 2.5). SBCE has been shown to be superior to push enteroscopy , abdominal computed tomography, abdominal magnetic resonance and angiographic studies [15–18], and as good as balloon-assisted small bowel enteroscopy [19], with diagnostic yield between 39 and 90 % [20]. Moreover, the rate of rebleeding in patients with occult GI bleeding and negative SBCE was found to be significantly lower (4.6 %) compared with those with a positive SBCE (48 %) [21].
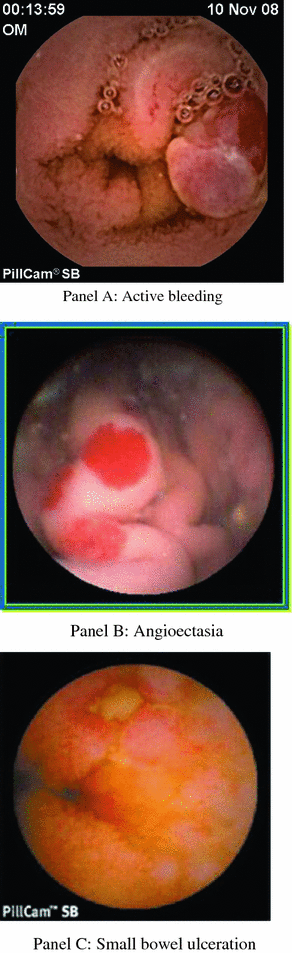

Fig. 2.5
Causes for small bowel obscure bleeding
This information will be covered in detail in the chapter on PillCam small bowel.
2.1.4 Crohn’s Disease
SBCE is an important tool both in the diagnosis and in the follow-up of Crohn’s disease. It is used to establish the diagnosis, to assess disease extent, severity, and disease activity, and to assess mucosal healing post-therapy (Fig. 2.6).
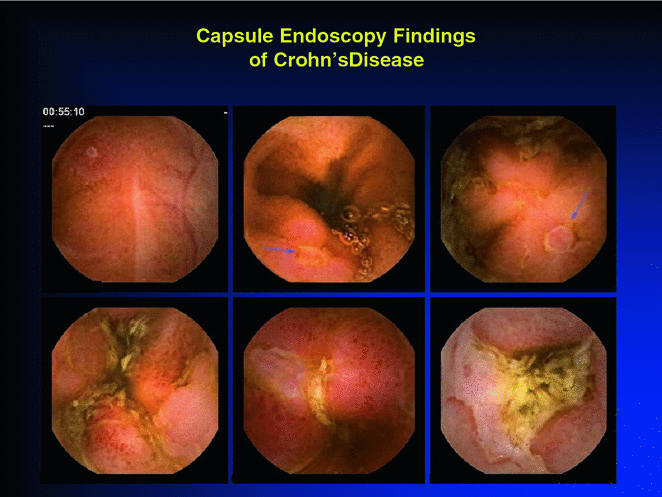

Fig. 2.6
Small bowel Crohn’s disease
SBCE has a high diagnostic yield in suspected Crohn’s disease . Moreover, for both known and suspected Crohn’s disease , SBCE was found to have a better incremental yield (ranging between 15 and 44 %) compared with other modalities, including small bowel follow-through, computed tomography, MRI, ileo-colonoscopy, and push enteroscopy [21]. Increase in the diagnostic yield of SBCE can be achieved by selecting patients with high pretest probability such as those with perianal disease and negative work-up, using the international conference on capsule endoscopy (ICCE) selection criteria and/or patients with high fecal calprotectin level.
SBCE may alter disease management of patients with known Crohn’s, by assessing mucosal healing after medical therapy. SBCE is the only method, except for double-balloon enteroscopy , to accurately assess small bowel mucosal healing. SBCE was also found to be clinically useful for categorizing patients with indeterminate colitis, although negative SBCE study did not exclude further diagnosis of Crohn’s.
The rate of SBCE retention in patients with suspected Crohn’s disease is similar to the general population (1.4 %), but retention rates of more than 8 % were reported in patients with established Crohn’s disease.
2.1.5 Small Bowel Tumors
The introduction of SBCE had resulted in doubling the rate of diagnosis of small bowel tumors to 6–9 % of patients undergoing SBCE for various indication s, obscure GI bleeding being the most common indication . More than half of the tumors diagnosed were malignant. Adenocarcinoma is the most common malignant tumor, followed by carcinoids, lymphomas, sarcomas, and hamartomas [22]. Gastrointestinal stromal tumor s are the most frequent benign neoplasm (32 % of all cases). Melanoma is the most common tumor metastasizing to the small bowel, although metastases derived from colorectal cancer and hepatocellular carcinoma have also been reported. Tumors are located most frequently in the jejunum (40–60 %), followed by the ileum (25–40 %), and the duodenum (15–20 %). Small bowel tumors can be easily missed due to the predominant submucosal and extraluminal location of the tumors. Specific indexes and scales were developed for improving the detection rate of small bowel tumors, including the Smooth Protruding Index on Capsule Endoscopy (SPICE score) and an automated scale using multiscale wavelet-based analysis [23, 24].
More details will be provided in the chapter on PillCam SB.
2.1.6 Celiac Disease
SBCE has a role in both the diagnosis of celiac disease and in the evaluation of gluten refractory celiac disease (Fig. 2.7). SBCE provides high-resolution magnified view of the mucosa, easily identifying the endoscopic changes found in celiac such as scalloping, mosaic pattern, flat mucosa, loss of folds, and nodularity. In a recent published meta-analysis, SBCE had an overall pooled sensitivity of 89 % and specificity of 95 % for identifying celiac disease [25]. In gluten non-responsive celiac disease , SBCE can be used for investigating the small bowel for tumors (enteropathy-associated T-cell lymphoma and adenocarcinoma ) and ulcerative jejuno-ileitis (Fig. 2.7).
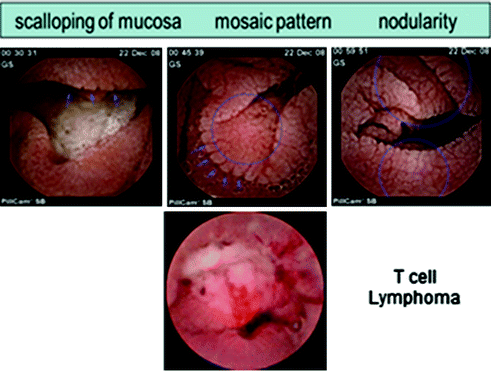

Fig. 2.7
Typical PillCam SB findings in celiac disease
2.1.7 Inherited Polyposis Syndromes
SBCE was shown to be effective tool in detecting small bowel polyps in Peutz–Jegher syndrome. It is especially effective in demonstrating small- and medium-size polyps. However, large polyps are sometimes only demonstrated partially, and polyp location is not accurate [26]. The duodenum is a potential pitfall as the capsule passes it very fast and thus may give false-negative results. The new SB3 SBCE may improve that with its six frames per second mode. Coupling of SBCE with double-balloon enteroscopy and polypectomy may offer an ideal method of follow-up and treatment of these patients, possibly avoiding surgery.
Another indication for SBCE in this setting is familial adenomatosis polyposis (FAP ) in which one may find patients with duodenal polyps, as well as small bowel polyps. However, the major papilla is not demonstrated effectively, and complementary examination with a side-view duodenoscope is mandatory.
2.1.8 Monitoring Effects and Side Effects of Drugs
SBCE can be used to monitor deleterious effects of drugs such as NSAIDs on small bowel mucosa. Lesions that can be found in these patients include erythema, erosions, small ulcerations, and weblike strictures. SBCE can be used to monitor the effect of drugs used to protect against NSAIDs-induced small bowel injury, to monitor the small bowel mucosal appearance in transplanted patients, to manage graft versus host disease, and, possibly, to monitor mucosal healing of small bowel Crohn’s disease after various medical treatments.
2.1.9 Capsule Retention
Capsule retention is the major complication of SBCE . Very rarely this may end in bowel obstruction /perforation. High risk of retention occurs in patients on NSAIDs , with known Crohn’s, with radiation enteritis , or with small bowel tumors. Normal prior radiological examination does not always protect from having capsule retention . Once retention is diagnosed (capsule not excreted 2 weeks after ingestion), endoscopic (balloon-assisted enteroscopy ) or surgical removal was shown to be effective. The intervention not only allows removal of the capsule, but also allows the offending abnormality.
2.1.10 Esophageal Video Capsule Endoscopy
In 2004, Given Imaging developed an esophageal video capsule (PillCam ESO ) as a noninvasive device for the examination of the esophagus. The second-generation esophageal capsule, the PillCam ESO2 (Given Imaging , Yokneam, Israel), was FDA-approved for marketing in 2007 (Fig. 2.8). The esophageal capsule endoscope (ECE) is a 26 × 11 mm capsule that differs from the SBCE in a few parameters: It has optical domes on both sides, the frame rate is much faster (9 frames from each side versus 2), a wider angle of view (169 vs. 156°), more advanced optics (3 lenses), and a shorter battery life of up to 30 min, all aimed to address the very short time (<2 s) of esophageal transit as well as the necessity to demonstrate the esophageal–gastric junction, where most of the esophageal pathology is located. It works for approximately 30 min and then shuts off and passed through the intestine via peristalsis and is naturally excreted. As in PillCam SB 3 system, or PillCam Colon2, real-time viewing is feasible.


Fig. 2.8
PillCam ESO capsule endoscope
Procedure: Prolongation of the transit time of the capsule has been achieved by an alteration of the capsule ingestion technique, using the simplified ingestion procedure (SIP) (Fig. 2.9), where the patient swallows the capsule after at least 3 h of fasting, lying in the right lateral position while sipping 15 mL of water every 30 s through a straw [27]. The procedure requires up to 5 min in an unsedated patient. Thus far, no other esophageal capsules are in the market. Competition includes attempts to attach a string to a Given Imaging small bowel capsule, the Given Imaging magnetic capsule, and the Olympus gastric capsule which are maneuvered with a joystick (Fig. 2.10).
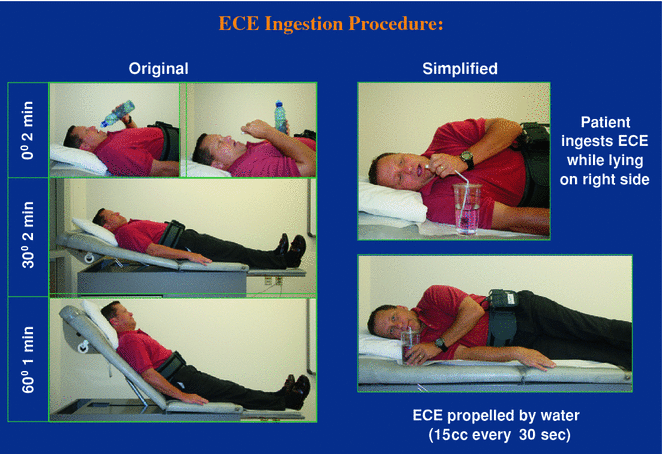

Fig. 2.9
Ingestion procedure for PillCam ESO capsule endoscope
Indications for ECE:
Screening for Barrett’s esophagus
Surveillance of esophageal varices in patients with portal hypertension.
ECE is safe, well tolerated, and reported to be preferred by patients to unsedated EGD. ECE was found to have variable sensitivity and specificity for the detection of GERD -related complication s. Few studies reported very high specificity and sensitivity for the detection of erosive esophagitis and Barrett’s esophagus (Fig. 2.11) [28, 29], while others found much lower rates of sensitivity and specificity. A recent meta-analysis of seven studies involving 446 patients, ECE was found to have a sensitivity of 86 % and specificity 81 % in detecting esophageal varices (Fig. 2.12) [30].
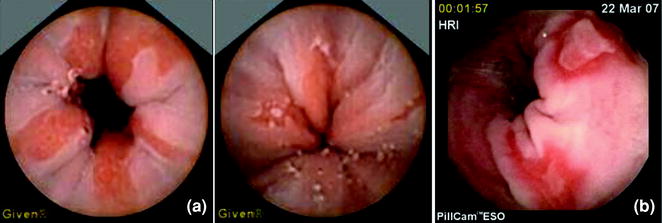
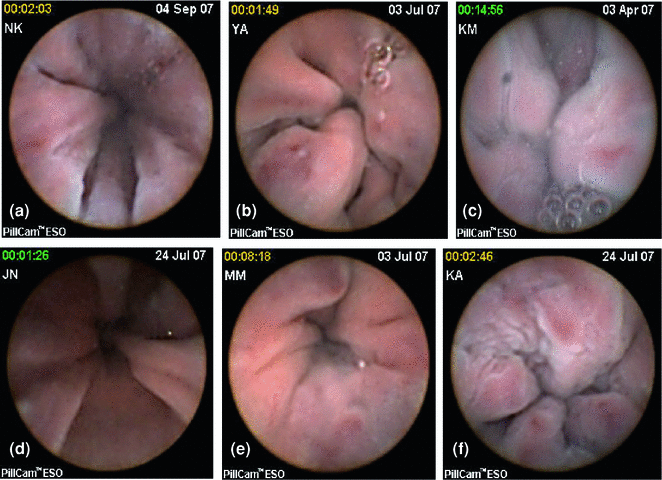

Fig. 2.11
PillCam ESO pictures of Barrett’s (a) and esophagitis (b)

Fig. 2.12
PillCam ESO pictures of esophageal varices. a–f Images of Esophageal varices taken with Pillcom ESO
Further details will be given in the chapter on Esophageal Capsule Endoscopy.
ECE may be used as an alternative to conventional upper GI endoscopy for the diagnosis of varices in complex patients with portal hypertension. It is most useful in certain patient groups: patients who poorly tolerate endoscopy or who have significant comorbidity, thus increasing the risks of repeated endoscopy, and patients with high risk of variant Creutzfeldt–Jakob disease.
Although the major innovations and technological advancement, at this point of time, ECE is not recommended as initial screening tool for the mentioned conditions, mainly due to the lower cost and higher availability of upper endoscopy.
2.1.11 Colon Capsule Endoscopy
Colon capsule endoscopy (CCE) (Given Imaging Ltd., Yokneam, Israel) was introduced in 2006 for the diagnosis of colonic pathologies, mainly polyps and tumors. In 2009, it went through major upgrading when the second generation of the capsule was introduced (Fig. 2.13). The second-generation capsule is slightly larger than the SBCE (31 × 11 mm) and has two camera domes with an adaptive frame rate of 4–35 frames per second, a 172° view angle for each camera, and longer life of up to 11 h due to the addition of a third battery and advanced engineering techniques. As mentioned, the frame rate can reach up to 35 frames per second depending on the capsule movement speed in the colon and is determined using the revolutionized adaptive frame rate technique via a cross talk between the capsule and the data recorder (DR3). This new recorder is endowed with artificial intelligence that communicates with the capsule, as well as with the patient by beeping and vibrating when the capsule leaves the stomach and displaying on the LCD screen a message that informs the patient to ingest a booster laxative which will accelerate the passage of the capsule through the small bowel.


Fig. 2.13
PillCam Colon2 capsule endoscope
Procedure: As in colonoscopy, bowel preparation is compulsory in order to achieve adequate mucosal visualization. This is done using a strict preparation that includes liquid diet on the day prior to capsule ingestion, two doses of 2 l of PEG solution (on the evening prior to ingestion and on the morning of the capsule ingestion), as well as propulsive agents to enhance capsule movement in the small bowel and advance it to and through the colon, while the battery is still working.
The main indication for CCE is colonic polyp detection (Table 2.1, Fig. 2.14). Colonic screening programs in moderate- and high-risk groups reduced the incidence, morbidity, and mortality due to colorectal carcinoma. However, compliance rates to colonoscopy screening programs are hampered due to fear of the invasiveness and possible complication s. CCE allows visualization of colonic mucosa with a minimally invasive procedure using no sedation, insufflation, or radiation and a practically complication -free method for colorectal screening . Because noninvasive colorectal imaging tests cannot provide a histological diagnosis, morphological criteria (i.e., polyp/mass ≥6 mm in size, or ≥3 polyps) are accepted as surrogate markers of advanced neoplasia. The average sensitivity of the first generation of CCE for significant findings (≥6 mm size, or ≥3 polyps irrespective of size) was relatively low, but it significantly improved with the use of the second-generation CCE (49).
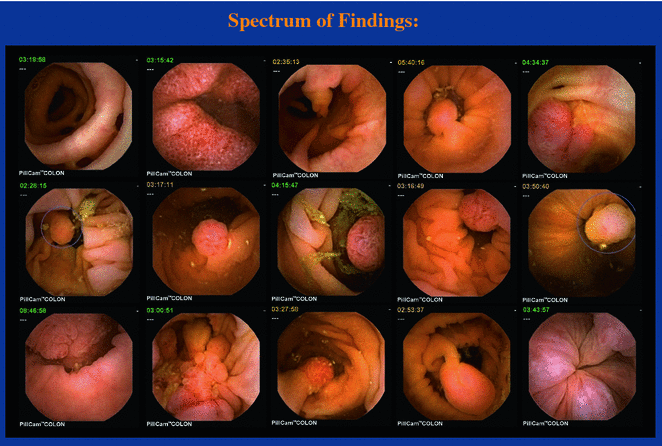

Fig. 2.14
Pathologies found with PillCam Colon2 capsule endoscope
Indication s: The latest guidelines published in 2012 by the European Society of Gastrointestinal Endoscopy (ESGE) [31] state that:
CCE is feasible and safe and appears to be accurate when used in average-risk individuals.
In patients with high risk for colorectal carcinoma in whom colonoscopy is not possible or not feasible, CCE could be a possible study.
CCE is also a feasible and safe tool for visualization of the colonic mucosa in patients with incomplete colonoscopy and without stenosis.
Another possible indication for CCE is in the diagnostic work-up or in the surveillance of patients with suspected or known inflammatory bowel disease (IBD), especially ulcerative colitis . Further details can be found in the chapter on Colonic Capsule Endoscopy .
2.1.12 Summary
Since its introduction almost 13 years ago, the clinical indication s for the use of capsule endoscopy have widened considerably. Capsule endoscopy has been proven to be a useful minimally invasive tool in the exploration of the entire gastrointestinal tract, allowing visualization of previously inaccessible parts and achieving worthy satisfaction from both physicians and patients. New indications and future possibility to control the capsule movement enabling new possibilities for diagnosis and targeted therapy will evolve with the future technologic advancement.
2.2 EndoCapsule
The EndoCapsule (Olympus , Tokyo, Japan) is a video capsule endoscopy for the small intestine using a charge-coupled device sensor instead of a CMOS to acquire images (Fig. 2.15). Launched in Europe in 2005, EndoCapsule obtained FDA clearance in 2007 [33]. The EndoCapsule consists of a camera, light source, transmitter, and batteries. Once the capsule is activated and swallowed by the patient, it begins transmitting images of the digestive system to a receiver worn by the patient. After the examination, the patient returns the receiver to the physician or a nurse who can download all images to a computer and find the abnormalities in small intestine (Fig. 2.16).
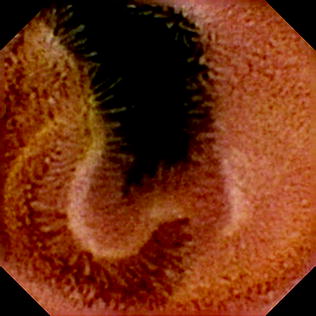

Fig. 2.16
Small intestinal villi detected by the Endocapsule . Reprint with permission from Cave et al. [36]
2.2.1 Special Characteristics [34]
1.
High-resolution CCD
2.
Smart Recorder: It combines a receiver and viewer in a compact and easy-to-handle unit, allowing the physician to playback and capture images any time during the procedure.
3.
3D Track Function: That function offers intuitive operation, showing capsule location to help you decide what approach should be taken for subsequent procedures.
2.2.2 Preparation
The bowel preparation of Endocapsule examination includes a 12-h fast prior to the procedure, the administration of 2 l of polyethylene glycol (PEG) solution in the evening and 1 l 30 min before the procedure.
2.2.3 Clinical Studies
In a British retrospective cohort study, 70 patients performed Endocapsule examination using either overview with express-selected (ES) or overview with auto-speed-adjusted (ASA) modes. The ES-mode software eliminates images with no significant changes (compared with the previous frames) in the video. And the ASA-mode software speeds up the fps of the CE video when detecting repetitive images. Among 40 (57 %) patients found with clinically significant findings, 32 (80 %) were recognized with overview function alone, while 39 (97.5 %) were recognized with overview function plus ES or ASA modes. The average reading time for overview with ES mode (19 ± 5 min) was significantly less than for overview with ASA mode (34 ± 10 min) (p = 0.001). These new playback systems can efficaciously reduce reading times of CE but need further evaluation in prospective multicenter studies [35].
Cave et al. carried out a multicenter randomized comparison of the Endocapsule and the PillCam SB in the USA. Results showed the positive percent agreement of 70.6 % and a negative percent disagreement of 82.4 % with an overall agreement of 74.5 %. The overall agreement was 74.5 % (38/51) with a κ of 0.48 and P = 0.008. The study demonstrated that Endocapsule had a similar diagnostic yield and better image quality compared with PillCam SB [36].
In another randomized head-to-head comparison study in Austria, 50 patients were randomly assigned to swallow either the MiroCam first, followed by the EndoCapsule 2 h later, or vice versa. The MiroCam and EndoCapsule devices were not statistically different with regard to their rates of complete small bowel examinations (96 vs. 90 %) or diagnostic yield (50 vs. 48 %). However, the findings were concordant in 68 % only (kappa = 0.50). The combined diagnostic yield was 58 % [37].
2.3 OMOM Capsule Endoscopy Platform
2.3.1 Overview
The creation of capsule endoscopy provides a new method for the visualized diagnosis of digestive diseases. It fixes the deficiency of the visualized diagnosis of small bowel disease s and brings a development direction of noninvasive, convenient, safe, and comfort diagnosis.
OMOM capsule endoscopy system is developed by Chongqing Science and Technology (Group) Co. Ltd. Comparing with other similar products, the unique feature of duplex multichannel communication mode has largely increased the controllability and convenience in its clinical use. Through the verification of clinical application, the product has equal validity and yield rate comparing with other similar products from overseas in the diagnosis of small bowel disease s, such as obscure GI bleeding, Crohn’s disease , small bowel tumor, and small bowel polyp [39–41].
Since the first generation of OMOM capsule endoscopy successfully created in 2004, Chongqing Jinshan Science and Technology has been dedicated to provide comprehensive solutions in the diagnosis of digestive diseases. Based on the first generation of capsule endoscopy, the company has developed various new capsule endoscopy products according to different clinical uses, such as controllable capsule endoscopy, storable capsule endoscopy, and CCE, in which it can provide safe, noninvasive, comfort, and convenient visualized diagnosis for the whole digestive tract.
After nearly 10 years of development , Chongqing Jinshan Science and Technology in the field of digestive medical area has launched a series of high-end products according to different clinical uses, in which they are able to provide accurate diagnosis of digestive tract disease with comprehensive and personalized solutions. The following article will describe in detail about the application range, product formation, functions, and features of the products.
2.3.2 Small Bowel Capsule Endoscopy
OMOM small bowel capsule endoscopy is mainly used for visualized diagnosis of small bowel disease s. It is a new diagnosis method which is noninvasive, painless, safe, and comfort. After swallowing the capsule, it will pass through esophagus, stomach, duodenum, jejunum, ileum, and colon and finally expel from human body naturally by digestive tract peristalsis. The capsule will continuously capture images of the GI tract during its movement process and transmit real-time image data wirelessly to the external image recorder. After the monitoring process, doctors can replay and analyze the saved images through the image workstation and finally make diagnosis of the gastrointestinal illness.
Small bowel capsule endoscopy system is mainly comprised of three parts: capsule, image recorder, and image workstation (Fig. 2.17), and functions of each part are described below:


Fig. 2.17
Small bowel capsule endoscopy system formation
Capsule: Capturing real-time image of GI tract and transmitting image wirelessly to the external image recorder; meanwhile, it is able to receive control signal from the image recorder to adjust working parameter.
Image recorder: Receiving and saving digital images from the capsule; also, it is able to send control signal to adjust the working parameter of the capsule.
Image workstation: Man–machine interactive operation platform can monitor the working condition of the capsule in real time and adjusting its working status. It is able to download and replay image data from the image recorder, assisting doctors to make diagnosis.
Indication s:
1.
GI hemorrhage, with no positive finding in upper and lower GI tract endoscopic examination;
2.
Small intestine imaging anomaly suggested by other examinations;
3.
Any type of IBD s, excluding bowel obstruction ;
4.
Unexplained abdominal pain and diarrhea;
5.
Small intestine tumor (benign, malignant, carcinoid, etc.);
6.
Unexplained iron-deficient anemia.
Contradictions:
1.
Patients who are confirmed (or suspected) to suffer from digestive tract malformation, gastrointestinal obstruction, gastrointestinal perforation, stenosis, or fistula;
2.
Patients implanted with pacemaker or other electronic devices;
3.
Patients suffering from severe dysphagia ;
4.
Patients suffering from acute enteritis or severe iron deficiency, for example, bacillary dysentery at active phase and ulcerative colitis at acute phase, particularly for patients suffering from fulminant diseases;
5.
Patients allergic to polymer material;
6.
Use with caution for patients below 18 and above 70 and for psychopath;
7.
Pregnant woman.
2.3.2.1 Features
Pioneer of duplex communication
It supports duplex data transmission between the capsule and the image recorder. The real-time monitoring function, which can check the captured images in real time during the examination, can able to make intuitive judgment about the location of the capsule within the GI tract. At the same time, it can control the parameters of the capsule, such as capture frequency, brightness, and exposure, in order to extend the monitoring time (Fig. 2.18). This function has been widely spread in clinical use, and it can increase the completion rate of small bowel examination up to 100 % [42, 43].
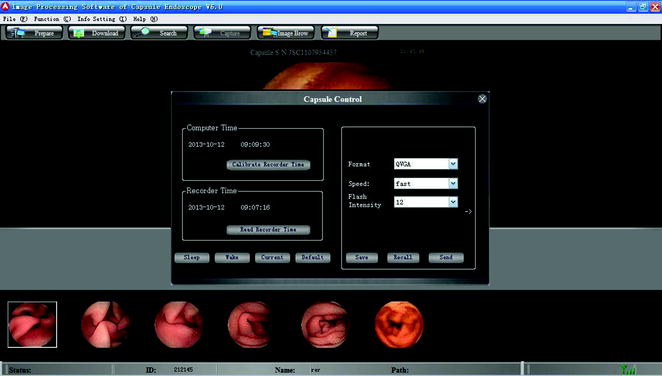

Fig. 2.18
Real-time monitoring and capsule working parameter adjustment
Unique multichannel mode
OMOM capsule endoscopy system supports simultaneous activation of multiple capsules at the same place, without interference between each of the capsules. Currently, OMOM capsule has 10 channels, which means it can undertake 10 patients simultaneously at the same location in the hospital without interference between each other. The image workstation can simultaneously monitor images from four capsules in real time (Fig. 2.19).
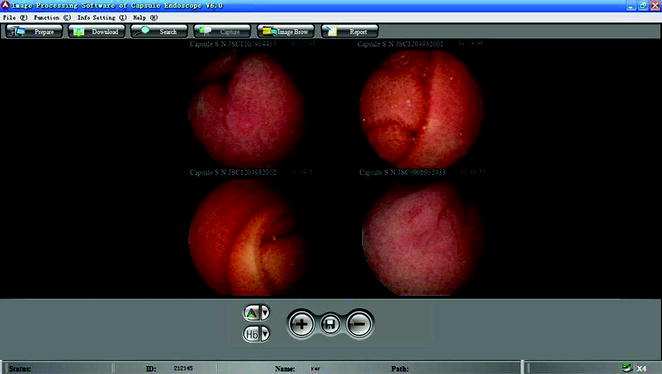

Fig. 2.19
Real-time monitoring of four capsules
Unique wireless USB monitoring
The wireless USB monitor is a convenient tool. It enables wireless communication, real-time monitoring, and capsule working parameter adjustment between the image recorder and the image workstation.
2.3.2.2 Clinical Application
Since 2005, OMOM capsule endoscopy has been used in clinic for over 8 years, and it has completed over 1 million samples. Clinical contrast study shows that OMOM capsule endoscope comparing with PillCam SB by Israeli company Given Imaging has no significant differences in the diagnosis of small bowel disease s, such as obscure GI bleeding, vascular malformation , small bowel tumor, small bowel polyp , and Crohn’s disease [44]. In addition, during the clinical use of OMOM capsule, its special feature of duplex communication function that enables real-time adjustment of image capture frequency can achieve 100 % completion rate of small bowel examination [42].
In 2,400 patients who had OMOM capsule examination [45], the diagnostic yield of small bowel diseases was 47.7 %. In all findings of small bowel, 28.1 % was vascular malformation, 18.9 % small bowel tumor, 10.4 % polyp, 7.9 % Crohn’s disease, 15 % mucosa injury and ulcers, 5.2 % bleeding, 11.3 % parasite, diverticulum, and so on. Comparing with traditional clinical methods such as GI radiography and CT, OMOM capsule endoscopy can provide more intuitive and clear images of small bowel, which is able to significantly increase the complete small bowel examination rate (CSER) and yield rate, and also, it provides more safety and reliability [46, 47]. At the same time, OMOM capsule endoscopy can incorporate other diagnostic methods such as double-balloon endoscopy in clinical use. It can further improve the CSER and yield rate, and it can help to confirm the lesion position and features prior to the small bowel surgery which is efficient to lower the risk and difficulty of the surgery, thus improving the surgery succession rate [48, 49].
2.3.3 Controllable Capsule Endoscopy
OMOM controllable capsule endoscopy is developed based on the small bowel capsule endoscopy. It can not only be used for visual diagnosis of small bowel, but can also achieve movement and angle control within the stomach. After swallowing the controllable capsule into the stomach, an external controller can control the capsule movement, posture, and angle from outside the body, which makes stomach examination controllable and comprehensive. After the stomach examination, the capsule will enter duodenum, jejunum, and ileum through GI tract peristalsis, and after the complete visualized examination of small bowel, it will pass through colon and be expelled from the body naturally. The capsule will continuously capture images within the stomach and small bowel during the examination, and the images will be transmitted and stored into the external image recorder wirelessly. After the examination, doctors can analyze the images and make diagnosis through the image workstation. The controllable capsule endoscope has solved the problems of ordinary capsule when undertaking stomach examination, such as large blind spot, insufficient observation, and high misdiagnose rate. It provides a painless, noninvasive, safe, and comfort method for stomach examination. Clinical study shows that the controllable capsule can achieve comprehensive examination of stomach fundus, stomach antrum, stomach corner, and stomach body with high detection rate and low misdiagnose rate [50, 51].
Controllable capsule endoscopy system is comprised of four parts: capsule, image recorder, image workstation, and controller (Fig. 2.20).


Fig. 2.20
Controller
2.3.4 Storable Capsule Endoscope
Storable capsule endoscope uses a large capacity storage module instead of traditional data transmission module. The captured images will be stored within the internal capsule memory module.
The advantages of storable capsule endoscopes are as follows: Patients do not need to wear an image recorder after swallowing the capsule, and they only need to be aware of the time of expelling the capsule from the body and collecting it. Then, a unique data reading and image viewing tool is used to process the images in order to make an analysis.
The storable capsule endoscope is disposable. The large capacity storage module contains 8 GB of memory which can store over 120,000 images. The working duration of the capsule reaches 15 h.
Storable capsule endoscope system is comprised of three parts: capsule, data reader, and image workstation.
Storable capsule endoscope is mainly used for the diagnosis of small bowel disease s such as unknown abdominal pain , GI hemorrhage, small bowel tumor, and Crohn’s disease .
2.3.5 Colon Capsule Endoscope
Colon capsule endoscope is a painless, noninvasive, safe, and comfortable diagnose method specially designed for colon disease. According to the physiological structure of colon and based on traditional capsule endoscope, it augmented more features such as capsule controlling, position measurement, posture measurement, and adjustment. It can achieve to control the movement, posture, and position of the capsule within the whole colon. Colon capsule can be entered to the colon through swallowing or anus insertion. The movement of the capsule can be fully controlled by the external controlling device. The capture images will be transmitted to the control panel in real time wirelessly, and adjust the capsule posture and angle to ensure the comprehensiveness and reliability of the examination. Therefore, comprehensive diagnosis of the whole colon can be achieved.
Colon capsule endoscope system is comprised of three parts: colon capsule, controlling device, and control panel (Fig. 2.21).

Fig. 2.21
Colon capsule endoscope system formation
Colon capsule endoscope system is mainly used for the diagnosis of various colon diseases such as colon inflammation, ulcer, diverticulum, polyp , and tumor.
2.3.6 pH Capsule Wireless Monitoring System
Gastroesophageal reflux disease (GERD ) is a common digestive disease which affects 10–20 % of European and American population [52], and this ratio is relatively lower in Asia, but it has an increasing trend [53]. Clinical research shows that continuous pH monitoring within esophagus is the most effective way of diagnosing GERD [54]. OMOM pH capsule wireless monitoring system is mainly used to monitor the pH value inside the esophagus and make diagnosis of GERD through detecting the change in pH value. The pH capsule is sent and fixed on the mucosa of the esophagus through the catheter, and it will monitor the pH value within the esophagus with the sensor through 96 h of continuous examination. The monitored data will be transmitted to the external data recorder wirelessly, and the doctor can make diagnosis by analyzing the continuously monitored pH data parameter through the workstation after the examination is completed. The capsule will naturally drop off from the mucosa and finally expel from the body.
Clinical application research shows that OMOM pH capsule wireless monitoring system is safe and efficient for diagnosing GERD. Long continuous monitoring time can reflect the status of the gastroesophagus reflux, which leads to high GERD positive detection rate, and it can effectively evaluate the frequency and severity of the reflux [55]. Comparing with traditional pH monitoring method such as catheter-based monitoring and endoscopy, it has similar diagnosis effect, but with easier and more convenient clinical operation [56, 57]; also, long monitoring time of 96 h is not only effective for GERD diagnosis in the early stage, but also effective for assisting therapeutic decision in the later stage, and it evaluates the effectiveness of medical treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree










