, Mark Thomas1 and David Milford2
(1)
Department of Renal Medicine, Birmingham Heartlands Hospital, Birmingham, UK
(2)
Birmingham Children’s Hospital, Birmingham, UK
Abstract
In this chapter we explain:
How to interpret haematuria
How myoglobinuria causes acute kidney injury
The quantification of proteinuria
Nephrotic syndrome
How to diagnose urinary infection
Urine testing strips are simple and informative tools for investigating kidney diseases (Fig. 11.1). The assessment of a patient with possible kidney disease is incomplete without urinalysis.


Fig. 11.1
Colour patterns from urine testing strips or ‘dipsticks’. The chart on the left is used to interpret the colour changes on the strips on the right
Haematuria
Seeing blood in your urine is alarming. As little as 1 mL of blood makes a litre of urine look blood-stained, so the colour exaggerates the amount of blood being lost.
It is important to take a precise history of the appearance of the urine.
Is it bright red (fresh bleeding) or brick red (altered blood, see Fig. 11.2)?

Fig. 11.2
Brick-red urine from a patient with severe acute nephritis . It is opaque due to cells and casts, which form a sediment at the bottom
Are there clots (urothelial rather than glomerular bleeding)?
Is the whole stream red from start to finish (blood entered the urine above the bladder outlet)?
Does the urine start red and then clear (blood from the prostate or urethra washed out by urine from the bladder)?
Men with visible haematuria should have a digital rectal examination and prostate specific antigen serum level measured.
People aged 45 and over with unexplained visible haematuria without urinary tract infection or that persists or recurs after successful treatment of urinary tract infection should be investigated for bladder and kidney cancer. People aged 60 and over with unexplained non‑visible haematuria and either dysuria or a raised white cell count on a blood test should similarly be referred [1]. Being on warfarin or other anticoagulant is not an adequate explanation.
The dipstick test distinguishes between free haemoglobin and non-haemolysed red cells (Fig. 11.3). The test strip also reacts to myoglobin. If haemoglobin or myoglobin are released into the circulation they are filtered through the glomeruli and stain the urine dark. Haemoglobinuria occurs with immune-mediated or mechanical intravascular haemolysis (see Sect. “Kidney disease linked to causes of anaemia” in page 174). Myoglobinuria is discussed in below section “Myoglobinuria”.
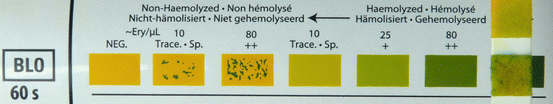

Fig. 11.3
Non-haemolysed microscopic haematuria detected on a urine dipstick test (right)
Dark urine with a negative dipstick test for blood can be caused by food dyes, laxatives containing phenolphthalein , rifampicin and, rarely, by porphyria and alkaptonuria .
How Red Cells Cross the Glomerular Filtration Barrier
If the glomerular filtration barrier is damaged in glomerulonephritis, protein as well as blood can appear in the urine. When combined with reduced GFR and high blood pressure, this constitutes the nephritic syndrome. If the barrier is weakened but otherwise intact, red cells can appear in the urine without protein and with a normal GFR. This is called isolated microscopic haematuria.
Red cells squeeze through the fenestrations in the endothelial cells and cross into Bowman’s space. As they pass down the tubule they may become embedded in uromodulin (Tamm-Horsfall protein ) and appear in the urine as red cell casts .
Red cells are often damaged as they are forced through the glomerular filtration barrier and so have irregular shapes in the urine. Rarely, they have been caught on electron microscopy in the act of traversing the barrier (Patient 11.1) [2].
Patient 11.1: A Red Cell Caught in the Act
A 22-year-old woman underwent a renal biopsy to investigate persistent microscopic hematuria. There was no macroscopic hematuria, no proteinuria and serum creatinine was normal. There was no family history of kidney disease and no hearing loss.
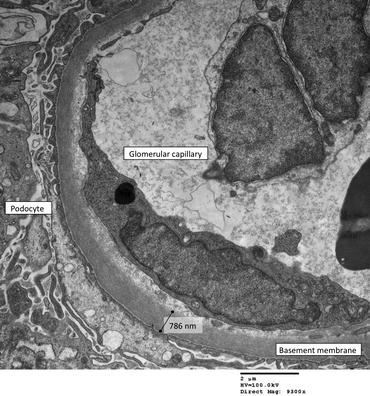
The glomeruli were normal under light microscopy and immunostaining for immunoglobulins and complement was negative. Electron microscopy showed the capillary walls to be thinner than normal. In one capillary loop, a red blood cell was found almost bisected by the glomerular capillary basement membrane (Fig. 11.4).
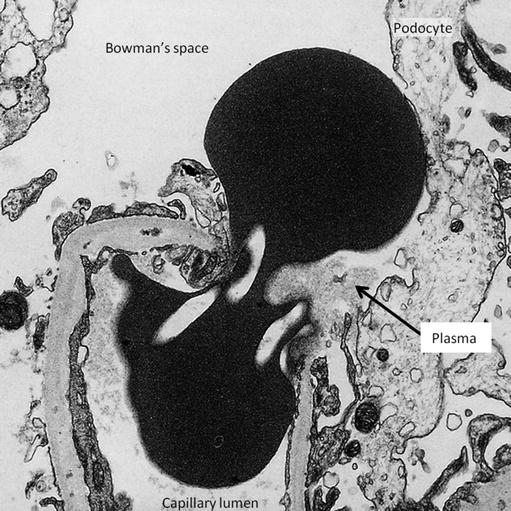

Fig. 11.4
A red cell passing upwards from the capillary lumen at the bottom of the picture through gaps in the cytoplasm of an endothelial cell. The podocyte foot processes have lost contact with the basement membrane and the cell body has been displaced by the red cell. Material similar in density to plasma (arrow) is present on the epithelial side of the membrane. This small amount of plasma would have been reabsorbed by the tubule, leaving the red cell alone to appear in the urine (Reproduced with permission from [2])
Similar pictures of the extravasation of red cells through gaps in endothelial cells have been produced experimentally in frog capillaries exposed to increasing amounts of pressure until they burst [3].
The Clinical Significance of Haematuria
Although seeing blood in your urine is alarming, haematuria is a less ominous sign than proteinuria in the context of glomerular diseases. For example, thin glomerular basement membrane disease (see Patient 11.1) causes haematuria but is a benign condition that does not lead to kidney failure.
The risk of a fit young person with only microscopic haematuria going on to develop end-stage kidney failure is increased but still very low – 34 versus 2 per 100,000 person years [4].
The commonest type of glomerulonephritis in adults is immunoglobulin A (IgA) nephropathy . It is characterised by the finding in the glomeruli of deposits of IgA. The IgA has abnormally low amounts of galactose bound to it but how this is linked to kidney damage remains unclear. Some patients present with visible (macroscopic) haematuria at the time of a sore throat – synpharyngitic haematuria. (The hematuria in post-streptococcal glomerulonephritis occurs about 2 weeks after the sore throat; see Sect. “Anti-streptolysin O (ASO) and anti-DNAse B antibodies” in page 204) This presentation has a largely benign prognosis, especially when there are repeated episodes of macroscopic haematuria [5].
The risk of kidney failure in patients with IgA nephropathy, and in glomerulonephritis generally, is higher if proteinuria is present, increasing in proportion to the amount of protein. High blood pressure and a reduced eGFR are also signs of a worse prognosis.
The abnormalities on the kidney biopsy in IgA nephropathy can be combined with clinical features to give a prognostic score [6]. Fibrosis in the interstitium is a stronger predictor than many of the glomerular changes. Patients 11.2, 11.3 and 11.4 illustrate the range of outcomes.
Patient 11.2: IgA Nephropathy – Recovery After a Worrying Presentation
Mr. Austin, aged 30 years, presented to hospital with abdominal pain and visible haematuria that had developed after an episode of diarrhoea and vomiting. Urine dipstick showed blood +++ and protein +++. Urine microscopy showed >500 red blood cells/mm3 but <5 white blood cells/mm3 and no malignant cells. Culture grew <104 organisms/cm3.
Full blood count was normal and serum creatinine = 86 micromol/L (1 mg/dL). The kidneys looked normal on ultrasound. Plasma immunoglubulins were:
Immunoglobulin IgG | 9.98 g/L | (Normal 6.00–16.00) |
Immunoglobulin IgA* | 3.96 g/L | (0.80–2.80) |
Immunoglobulin IgM | 1.01 g/L | (0.50–1.90) |
Two days after admission, his eGFR dropped to 56 ml/min/1.73 m2.
A renal biopsy showed 13 largely normal glomeruli but an increase in cells in part of one glomerulus – a segmental glomerulonephritis. Immunofluorescent staining showed many granular deposits of IgA and complement C3 in the mesangial cells of all glomeruli (Fig. 11.5). The mesangial cells lie between the glomerular capillaries and are attached to their basement membrane. They are phagocytic and take up deposits containing IgA and C3 that are trapped by the filtration barrier. These appearances are typical of IgA nephropathy.
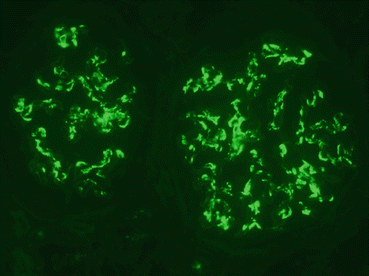

Fig. 11.5
Immunofluorescence microscopy showing positive staining for IgA in the glomerular mesangium. ×400
Occasional tubules contained red blood cell casts (Fig. 11.6).
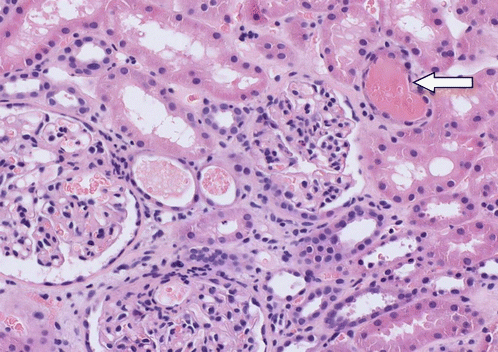

Fig. 11.6
Section through kidney cortex showing glomerular changes of IgA nephropathy, including mesangial hypercellularity and thickening. A number of tubules contain red cell casts, one of which is occluding the lumen (arrow). Haematoxylin and eosin ×200
The acute kidney injury was attributed to the red cell casts obstructing the tubules [7]. eGFR recovered to its previous level over the following three months (Fig. 11.7).
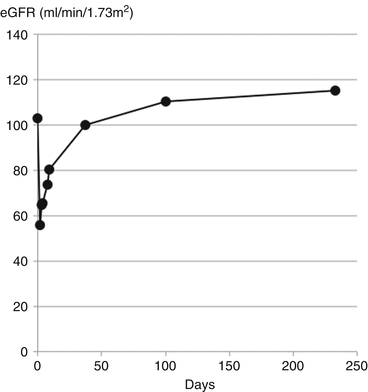

Fig. 11.7
Transient drop in eGFR due to red cell casts in IgA nephropathy
Three years later Mr. Austin was reviewed in clinic. His urine had been intermittently blood-stained, each time associated with vomiting and joint pain. His serum creatinine was the same as when he first presented – 86 micromol/L (1.0 mg/dL).
Patient 11.3: IgA Nephropathy – From Haematuria to CKD Stage G5 in 43 Years
Paul first presented in 1971 at the age of 22 years with fever, sore throat, frank haematuria and four episodes of loin pain. His blood pressure was high, ranging from 160/102 to 195/120 mm/Hg. The urine protein/creatinine ratio was 43.1 mg/mmol (380 mg/g) and a 24 h urine collection contained 0.68 g of protein.
A renal biopsy showed some sclerosed glomeruli and others with segmental proliferation consistent with IgA nephropathy. There were some fibrotic scars in the interstitium.
His blood pressure was difficult to control due to drug side effects. However, he remained very fit and twice ran the London marathon in under 4 h.
His kidney function declined over the following 43 years, charted over the last 18 years in Fig. 11.8.
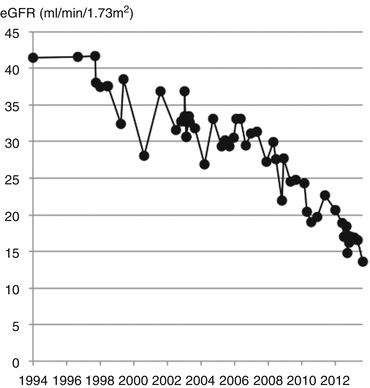

Fig. 11.8
Decline in eGFR at a rate of approximately 2 ml/min/1.73 m2/year due to IgA nephropathy
Patient 11.4: IgA Nephropathy – No Visible Haematuria but End-Stage Kidney Failure in 2 Years
Mr. Mitchell presented for an insurance medical examination at the age of 47. He was a heavy smoker. His urine was found to contain microscopic haematuria and heavy proteinuria, urine protein:creatinine ratio being 565 mg/mmol (4972 mg/g). His eGFR was 69 ml/min/1.73 m2.
A renal biopsy specimen showed expansion and increased cellularity of the mesangial region with interstitial and periglomerular fibrosis (Fig. 11.9).
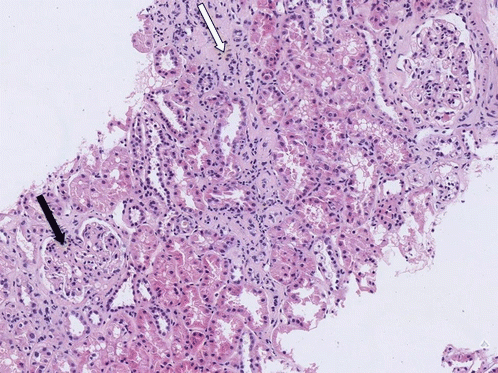

Fig. 11.9
Light micrograph showing expansion and hypercellularity of the mesangial region with glomerulosclerosis (black arrow). There is interstitial fibrosis and tubular atrophy, best seen as a vertical band of pale cellular tissue between the tubules in the middle of the section (white arrow) and around the glomerulus in the upper right corner. Haematoxylin and eosin ×100
Immunofluorescent staining showed the typical features of IgA nephropathy (Fig. 11.5).
His eGFR rapidly declined over the following 2 years (Fig. 11.10). Smoking may have accelerated the decline; smokers with IgA nephropathy are twice as likely to lose kidney function [8].
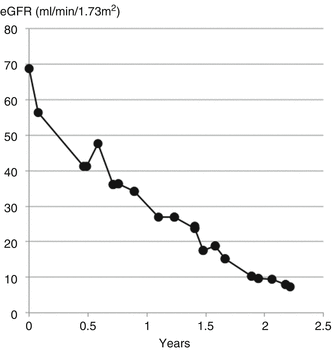

Fig. 11.10
Decline in eGFR at a rate of 30 ml/min/1.73 m2/year due to IgA nephropathy
He later had a successful kidney transplant.
Myoglobinuria
Myoglobinuria results from the breakdown of large amounts of muscle tissue. Physical damage can be due to compression from prolonged immobility or crush injury, ischaemia from embolism, electric shock or severe exercise induced by recreational drugs (Patient 11.5). Prescribed drugs, notably statins , and infectious or inflammatory diseases that cause myositis can cause muscle breakdown. Metabolic conditions such as McArdle disease , hypokalaemia or an enzyme deficiency make the muscles vulnerable to damage [9].
A number of mechanisms lead to acute kidney injury with myoglobinuria . Necrotic muscle swells up leading to hypovolaemia and shock. Myoglobin is concentrated in the tubules where it precipitates with uromodulin to form casts that obstruct the flow of filtrate. Increased uric acid production from muscle protein leads to uric acid casts , which are more likely to precipitate in the low urine pH caused by metabolic acidosis. And finally, the haem centre of the molecule is toxic to the tubular cells which reabsorb the filtered myoglobin.
The exposed sarcoplasm and myofibrils in damaged muscle release potassium and phosphate into the circulation and bind calcium, causing hypocalcaemia and cardiac arrhythmias. The damaged muscles can become calcified. The bound calcium is released during the recovery phase as the muscles heal, causing hypercalcaemia .
Patient 11.5: Rhabdomyolyisis, Myoglobinuria and Acute Kidney Injury
Ryan, a 23-year-old unemployed man, was admitted as an emergency having been found unconscious on the floor. He had lain there for at least 12 h. He had a history of epilepsy and alcohol dependency, and used intravenous heroin.
On arrival at the casualty department, the skin over his legs had already begun to blister from pressure damage, and his muscles were tense. He was oliguric and his urine was like cola (Fig. 11.11).
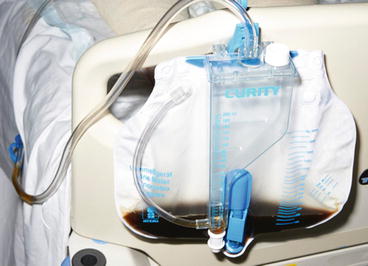

Fig. 11.11
Cola–coloured urine due to myoglobinuria
His serum creatinine had increased from 78 micromols/L (0.9 mg/dL) seven months previously to 172 (1.9) on arrival and 297 (3.4) 13 h later (stage 3 AKI; see Table 3.2). Serum creatine phosphokinase was >50,000 IU/L.
On arrival, his serum potassium was high (8.6 mmol/L, mEq/L) and calcium low (corrected for serum albumin = 1.78 mmol/L, 7.1 mg/dL) due to the release of potassium and binding of calcium by damaged muscle fibres.
Because the pressure within all the muscle compartments of his legs was extremely high, 100–125 mmHg, multiple fasciotomies were performed. The muscles were swollen and some visibly necrotic (Fig. 11.12).
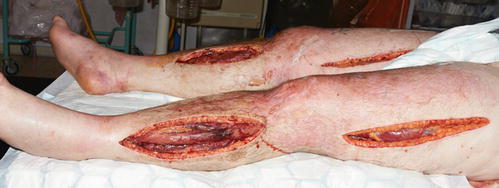

Fig. 11.12
Multiple fasciotomies revealing necrotic muscle. There is blistering of the skin by the right knee caused by prolonged pressure and oedema
Proteinuria
Normal glomerular filtration allows an estimated 3.3–5.7 g of albumin per day to pass into the proximal tubule. This is almost all reabsorbed and very little appears in the urine. If the filtration barrier is damaged, the amount of albumin and other proteins that pass into the tubule can overwhelm its absorptive capacity so that much more appears in the urine (Fig. 11.13).
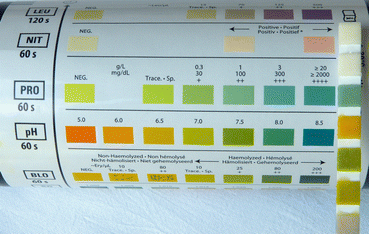

Fig. 11.13
Heavy proteinuria on a urine dipstick test (PRO +++)
Large amounts of albumin make the urine foamy . Normal urine can make froth if it is concentrated and rapidly urinated or if it contains large amount of semen due to retrograde ejaculation . Bubbly urine can be passed in patients with a vesicocolic fistula or infection with gas forming organisms.
Perhaps surprisingly, the passage of albumin across the filtration barrier is easier when the thickness of the glomerular basement membrane is increased (Fig. 11.14). Conversely, patients with thin basement membranes do not have proteinuria (see Sect. “How red cells cross the glomerular filtration barrier” in page 135). It is the structural integrity and the size and electrical charge of pores in the membrane rather than its thickness that determine how easily proteins can pass through.