Fig. 6.1
Typical ureteroscopic appearance of a small papillary low grade tumor in the renal pelvis
Accurate staging of upper urinary tract transitional cell carcinoma is challenging. CT evaluation alone has been shown inadequate for accurate staging, and the underlying ureteral musculature can seldom be safely sampled ureteroscopically. Fortunately, stage and grade correlate closely, allowing grade to help guide treatment options [15]. While cytology is specific, it is less sensitive because most low-grade lesions will be missed. Higher grade transitional cell carcinoma lesions are more likely to provide positive cytologic findings as the tumor cells have lost their cell-cell adhesion capabilities and demonstrate more easily identifiable morphologic changes. Direct ureteroscopic biopsy is highly sensitive but less specific. Thus, cytology and biopsy are complementary tools in diagnosing and grading these tumors.
Minimizing ureteral trauma during ureteroscopy is important to maintain excellent visibility, diminish trauma related erroneous lesions, and to decrease the risk of tumor seeding during ureteroscopy. Ureteroscope outer diameter has decreased over time, which has improved our ability to insert the ureteroscope without prior ureteral dilation [11]. This is critical because pre-stenting or ureteral dilation at the time of surgery creates iatrogenic trauma that can be difficult to distinguish from recurrent disease, particularly carcinoma in situ. As the invasiveness of ureteroscopy decreases, there will be fewer reasons to rely on radiologic studies when one can safely inspect and sample the urothelium of the upper urinary tract.
Patients with significant comorbidities make ureteroscopic inspection a higher risk endeavor. For these patients, retrograde ureteropyelography alone may be a reasonable alternative. In these patients, ureteroscopy can be reserved for further investigation of significant filling defects. There are no data showing improved survival with one screening method over another. Although it is known that small tumors will be missed with retrograde ureteropyelograms alone, it is not unclear if delaying treatment of these small recurrences until they are large enough to be detected on retrograde ureteropyelography will result in any additional harm to the patient. Nevertheless, direct ureteroscopic inspection is favored over retrograde pyelography and voided cytology in patients without prohibitive comorbidities.
6.3 Surveillance Protocols
As detailed, multiple surveillance options exist following endoscopic treatment of upper tract tumors. Bagley and colleagues sent a survey to members of the Society of Urologic Oncology, Endourological Society, and American Urological Association regarding treatment and surveillance strategies for upper tract tumors [21]. Seventy percent of respondents performed surveillance ureteroscopy. The remainder used excretory urography or retrograde pyelography without upper tract endoscopy. Time to initial surveillance ureteroscopy was split evenly between 3- and 6-months. None of the responders relied entirely on urinary markers.
At present there are no detailed, evidence-based guidelines on the ideal surveillance protocol, which is highlighted by the wide variety of practice patterns. One of the most detailed surveillance protocols has been outlined by the Jefferson University group [21]. Their institutional protocol consists of cystoscopy, retrograde pyelography and ureteroscopy every 3 months until the patient is disease free. Office cystoscopy is then performed at 3, 9, 15, and 21 months. Ureteroscopy in the operating room is performed at the 6, 12, 18 and 24 month mark. After the first 2 years, cystoscopy is performed every 6 months and ureteroscopy annually.
The American Urological Association has not released any guidelines pertaining to the management of upper tract urothelial tumors. The National Comprehensive Cancer Network (NCCN) Bladder Cancer Guidelines do provide specific management recommendations for upper tract tumors but their surveillance recommendations are limited (NCCN guidelines). The guidelines recommend follow-up ureteroscopy and upper tract imaging (intravenous pyelography, retrograde pyelogram, CT or magnetic resonance urography) at 3–12 month intervals. Decreased surveillance frequency over time for patients without evidence of recurrence is not addressed.
The European Association of Urology has published guidelines on urothelial tumors of the upper tract [23]. Following endoscopic treatment, the Guidelines recommend performing urinary cytology and CT urography at 3 and 6 months then annually for at least 5 years. Cystoscopy, ureteroscopy and in situ cytology is also performed at the 3 and 6 month mark, then every 6 months for 2 years, then yearly.
A summary of the two available sets of recommendations for surveillance of patients following treatment of upper tract urothelial carcinoma is presented in Table 6.1.
Table 6.1
Summary of Surveillance Guidelines from the NCCN and EAU
NCCN | EAU | |
|---|---|---|
Bladder | ||
Modality | Cystoscopy | Cystoscopy and cytology |
Frequency | every 3 months for 1 year, then increasing intervals | at 3 months, 6 months, then every 6 months for 2 years, then annually |
Upper tract | ||
Modality | IVP, CT urography, retrograde ureteropyelogram, ureteroscopy, or MRI urogram | Ureteroscopy |
Frequency | 3–12 months intervals | at 3 months, 6 months, then every 6 months for 2 years, then annually |
Other | +/− CT, +/− CXR | CT urography at 3 months, 6 months then annually for at least 5 years |
Table 6.2
Technique of Surveillance Ureteroscopy
1. Cystourethroscopy |
(a) Careful and complete |
(b) 30° and 70° lens |
2. Bladder lavage for cytology |
3. Remove cystoscope |
4. Rigid ureteroscopy |
(a) Direct insertion of ureteroscope without a wire |
(b) Leave wire through the ureteroscope to the level inspected |
(c) Remove rigid ureteroscope, leaving guidewire in place |
5. Flexible ureteroscopy |
(a) Over the previously placed wire |
(b) Inspect proximally from the level reached with rigid ureteroscope |
6. Systematic inspection of intrarenal collecting system |
(a) Fluoroscopy while irrigating with dilute contrast to ensure completeness |
7. Aspirate intrarenal urine for cytology |
8. Biopsy any suspicious lesions |
9. Lavage for cytology after biopsy |
10. Consider laser ablation of obvious urothelial carcinoma recurrence |
11. Remove flexible ureteroscope and stent as indicated |
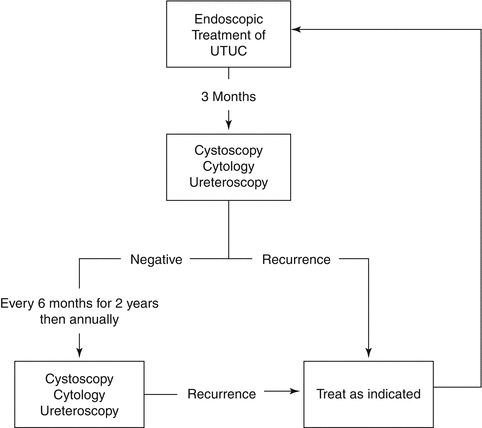
Our recommended surveillance protocol algorithm for these patients is presented in Fig. 6.2. In our protocol, the frequency of cystoscopy and upper tract imaging is based on the treatment of patients with low grade urothelial carcinoma, as this will make up the majority of patients treated endoscopically. There are very few recommendations in the NCCN and EAU guidelines regarding surveillance following nephroureterectomy. In the absence of any specific guidance, we recommend bladder recurrence screening with cystoscopy should be performed with a frequency based upon the grade of the tumor, and follow the same guidelines as those for patients treated for bladder cancer. Upper tract imaging of the contralateral upper tract can be performed with retrograde ureteropyelography or CT urography at 1 year, then every 1–2 years.