Fig. 1.1
Persistent sepsis and a fistula in formation are in evidence. While both ischioanal fossae were incised and connected with a draining penrose seton communicated through the superficial postanal space, the deep postanal infection was not addressed by this intervention
The root cause for persistent sepsis following a surgical drainage procedure is multifactorial. In the series above, infections classified as perianal had the highest rate of reoperations followed by ischiorectal, intersphincteric and supralevator abscesses. The observation is counter-intuitive. However, it is not difficult to understand. Many surgeons, particularly the less experienced focus much attention on the obvious topographic appearance of the perianal region. This tendency facilitates overlooking important elements in the history and physical examination of the patient. Most anorectal infections appear simple. However, they may represent the “tip of the iceberg” of a more complex infection.
The authors speculate on the legitimacy of the concept of loculated abscesses. It is a difficult concept to prove objectively. Multiple space infections, however, are well documented. Examples include secondary ischioanal infections from a deep post anal space or supralevator space primary. Supralevators may also appear as a perianal infection on topographic inspection.
While the percentage of persistent infections is relatively small, it is not a diminutive topic in clinical practice. It is seen commonly in diabetic patients even when appropriately drained. These patients often require a tincture of time on antibiotics, glucose control and local wound care (sits baths). Ongoing sepsis is also common in patients who undergo drainage of a secondary infection leaving the primary septic focus undrained. When in doubt, it is best to image the patient and proceed to the anatomically indicated drainage procedure in a formal operative setting.
Progression of Sepsis
The concept of “pus under pressure” allows dissection along tissue planes and into potential spaces. Cryptoglandular infections can also disseminate into the supralevator space through various routes as discussed in Part B. Once seeded, large collections can propagate to the psoas muscle or along the paraspinal musculature. The treatment of these complications must be control of the primary site of infection and drainage of the infection itself. Necrotizing fasciitis is a relatively uncommon complication of cryptoglandular disease seen in the setting of immunologically challenged individuals [3].
Recurrent Abscess
Recurrent abscess is a common finding with cryptoglandular disease. 25–50% of patients may experience recurrence of an abscess. Recurrence should be differentiated from persistent disease through a thorough history and delineation of the patient’s symptoms. Without a symptom-free interval, the abscess was likely not resolved from the initial treatment. True recurrent abscesses may or may not have an associated anal fistula. Recurrent infection is distinctly less common following definitive treatment of the abscess addressing the primary fistulous tract as compared to simple incision and drainage alone [4]. Definitive surgery also results in fewer surgeries overall vis-a-vis simple drainage [5].
Delayed Wound Healing
The perineum is normally a very well vascularized field. Redundant blood supply through the branches of the internal iliac arteries allow for excellent healing in most circumstances. Despite the possible frequent contamination from stool, these wounds will often heal quickly. Nutritional status and immune competence may mitigate wound healing. Robust packing of wounds has been demonstrated to delay wound healing [6]. Wounds rarely heal in the setting of previous radiation. Occasionally, non-healing wounds may be seen in the context of an underlying malignancy or osteomyelitis of a pelvic bone may be seen. Poorly optimized patients with human immune deficiency virus may demonstrate poor wound healing. However, they are generally symptomatically benefited from anorectal surgical interventions.
Wound Contraction Deformities
Contraction deformities or “step-offs” are the result of cruciate or vertical drainage incisions. Neither of the previous has been show to prevent premature wound closure. However, the effectiveness of radial incisions was reported to be 99.6% in one large series [7]. Radial incisional drainage was first described by Ayers in 1886 specifically emphasizing the prevention of contraction deformities [8].
Iatrogenic Fistula
Fistula may form following an anorectal abscess in 5–85% of cases [9]. An iatrogenic fistula can form following incorrect drainage of a complex abscess. Examples include a suprasphincteric fistula secondary to external drainage of an intersphincteric supralevator abscess. Conversely, extrasphincteric fistula result from internal drainage of an extrasphincteric supralevator abscess. Theoretically, iatrogenic fistula may result from definitive treatment of the abscess and primary fistulous trajectory simultaneously. However, the incidence of this latter complication is unknown. It is also important to note that complex fistula may form when simple drainage is performed on multi-space infections. Spontaneous drainage of an abscess can also produce complex fistulas.
Chronic Fistula
Conventional wisdom dictates that a certain proportion of patients with an acute anorectal infection will progress to the chronic phase with an anal fistula. To what degree a chronic fistula is preventable is not known. However, it is clear that drainage with definitive treatment of its primary fistulous component is associated with fewer operations subsequently for recurrent abscesses and fistula. The incidence of incontinence does not appear to increase with combined treatment [10]. Definitive treatment of infections involving the deep postanal space abscesses can prevent horseshoe, hemi-horseshoe, suprasphincteric, and extrasphincteric fistulas in most cases.
Incontinence
Anorectal infections and their treatment continue to put patients at risk for incontinence. Knoefel also note that recurrent infections are more likely to alter sphincter function than fistulotomy with drainage than simple drainage alone [11]. Prior to any intervention that may involve the sphincters, a surgeon should assess sphincter function in both a subjective and objective fashion. The patient should be queried as to their current function with control of stool and gas. Objectively the resting pressure and squeeze pressure of the anorectal ring should be assessed. This does not need to involve specific manometric testing but rather a digital exam by an experienced practitioner. In the acute setting this may be impossible due to pain from the infection. The physician can then document the perceived continence prior to the infection. If treatment for fistula is to be undertaken, preoperative assessment is essential prior to operative decision-making. The subjective portion of the patient’s continence may then be less reliable due to leakage from the fistula. Many patients may be unable to distinguish incontinence from fistula drainage in this setting. In the current state of the art, alterations in continence may result from both simple drainage as well as definitive treatment of abscesses with the fistulous components. Fortunately, it is an uncommon problem (1–2%) [10]. Moreover, these alterations are generally limited to gas and liquid incontinence of the majority. Full disclosure of the inherent risks, albeit small, is paramount in all cases.
Part II: Prevention Strategies
The prevention of untoward sequelae in the treatment of acute anorectal infections begins with a systematic clinical approach. Pain is the most common symptom. Its time in evolution and duration are important considerations. Rapid onset over a short duration is commonly seen with perianal infections. This feature is thought to be secondary to the dissection of pus through the tight fascial compartments of the corrugator cutis extensions of the sphincter muscles out toward the skin at the anal margin. Pain augmented on defecation is compatible with an intersphincteric infection secondary to the dissection of pus between the internal and external sphincters. Longer durations of symptoms favor infections occupying large spaces like the ischioanal fossa or multiple spaces (e.g., supralevator abscesses). Tenesmus and pain augmented with defecation are also characteristic of supralevator processes.
Visual inspection of the perianal region is important. However, it needs to be considered in the context of acute cryptoglandular obstruction as a chronologic sequence of inflammatory changes. Cellulitis generally precedes lymphedema and lymphatic obstruction. Fluctuance secondary to a frank abscess is a relatively late event in this sequence. Therefore, clinicians should not over interpret obvious topographic signs, as they may be harbingers of deeper seated infections. The absence of obvious clinical signs suggests an occult anorectal infection. Submucosal, intersphincteric, deep post anal space and supralevator abscesses fall into this category.
Digital rectal examination should also be systematic. Palpation of a boggy mass above the sphincters is the sine qua non of supralevator processes. Submucosal abscess begin in the anal canal extending proximally a short distance. Intersphincteric supralevators are palpable above puborectalis muscle. Bi-digital examination with the examiner’s index finger within the rectum and thumb over the ischioanal fossa identifies involvement of the latter space. Palpation of the anal canal may reveal an area of induration or a divot associated with the site of the primary cryptoglandular obstruction. Purulent discharge per anus has three possible explanations: (1) decompression of the abscess at the primary crypt of origin; (2) spontaneous decompression of a supralevator abscess into the rectum; and (3) proctocolitis. When formal exploration of an abscess is contemplated, the abscess should not be compressed until evaluation under anesthesia in order to optimize one’s ability to identify the primary crypt and fistulous trajectory.
The natural history of anorectal infections is mucocutaneous necrosis and spontaneous drainage by Providence unless intervened first by a competent physician. Longstanding neglected infections upon spontaneous drainage may produce undesirable consequences including complex fistula. Therefore, the time honored concept of early drainage remains as an operant fundamental concept.
The tetralogy of occult anorectal sepsis consists of pain, evidence of infection, a paucity of obvious physical findings and hyperesthesia often limiting the physical examination. It is commonly associated with submucosal, intersphincteric, isolated deep post anal and supralevator abscess. Imaging is invaluable in this setting with the intent to treat formally in the operative room setting.
A Practical Approach to Anorectal Infections
Cryptoglandular infection propagates by three routes: (1) superficial, (2) intersphincteric, and (3) transsphincteric. Extrasphincteric refers to a supralevator transsphincteric process. Superficial infections result in submucosal or subcutaneous abscess. They pose few problems if correctly diagnosed. While submucosal abscess are generally associated with normal perianal topography, they are palpable within the anal canal as a walnut-size soft and tender mass. Intersphincteric trajectories may present as perianal, an abscesses confined to the anal canal or with proximal extension as a supralevator abscess. Combinations in all three spaces are seen with an intersphincteric trajectory. Transsphincteric trajectories are the least common, but the most problematic. In its simplest configuration, it results in a primary unilateral primary ischioanal abscess. A posterior transsphincteric fistulous trajectory results in a deep post anal space abscess. Abscesses in this location may be isolated with negligible physical signs if seen early. However, they may propagate anterolaterally to produce a secondary ischioanal abscess. Bilateral propagation from the deep post anal space results in one important variant of a “horseshoe” presentation. Deep post anal space infections can dissect between the leaves of the pubococcygeus muscles that insert onto the sides of the coccyx. This trajectory produces a posterior extrasphincteric supralevator abscess. In its most unusual form, a deep post anal space abscess can propagate to both the supralevator and ischioanal spaces. The secondary ischioanal component can independently and simultaneously disseminate into the supralevator space along the medial aspect of the fascia of the obturator internus muscle. This scenario produces a combined extrasphincteric supralevator abscess from a deep post anal primary and ischioanal secondary infection. Ortega and colleagues have proposed a novel classification of supralevator abscesses based on these principles: type I intersphincteric, type II anterolateral extrasphincteric via the ischioanal fossa, type III posterior extrasphincteric from the deep post anal space, and type IV combined posterior and anterolateral extrasphincteric via the deep post anal and ischioanal spaces simultaneously [12] (Fig. 1.2).


Fig. 1.2
Novel classification scheme for supralevator abscesses. Type I—Intersphincteric. Type II—Extrasphincteric supralevator extension of a primary transsphincteric ischioanal abscess. Type III—Posterior extrasphincteric supralevator extension from a primary deep post anal space abscess. Type IV—Extrasphincteric supralevator extension from both the deep post anal space and the ischioanal space simultaneously (unilateral or bilateral)
The correct diagnosis and treatment of supralevator abscesses require the evaluation of three spaces: (1) supralevator, (2) ischioanal, and (3) the deep postanal. It is important to point out that more than half of supralevator abscesses of cryptoglandular origin have the deep postanal space as their primary site of infection. Figure 1.3 demonstrates the diagnostic and treatment algorithm required for successful management of supralevator infections.


Fig. 1.3
Algorithm for treatment of supralevator abscesses. Evaluation of three spaces is required: (1) supralevator, (2) ischioanal, and (3) deep postanal. Their inclusion or exclusion determines the correct intervention required to prevent recurrent or persistent sepsis as well complex fistula
Simple superficial low infections pose few problems in the short and long term. Deeper seated as well as infections occupying multiple spaces are distinctly different. The latter offer opportunities for the prevention of persistent, recurrent, and chronic sepsis (i.e., complex anal fistula). Therefore, horseshoe presentations, ischioanal, deep postanal, supralevator abscesses, and horseshoe presentations merit special consideration.
Ischioanal infections are the second most common type of infection following perianal abscesses. In contrast, their presentation is longer in evolution and their size is greater in lateral extension. Few topographic signs may be present early in their evolution. However, a palpable fullness between the examiner’s index finger and thumb may be appreciated. In their most common later presentation, a large area of cellulitis often extending several centimeters outside the anal margin extending over the gluteal tissues may be seen. They are often drained as a bedside procedure. This common practice merits reconsideration. Primary ischioanal abscesses represent a direct transsphincteric process to one isolated fossa. Secondary ischioanal infections originate in the deep postanal space and then propagate to one or both ischioanal fossae. This bilaterally may be symmetrical, asymmetrical, or delayed. For all these reasons, the authors recommend imaging of ischioanal abscesses in order to facilitate definitive management. This strategy appears to be most reasonable for the prevention of persistent and recurrent sepsis as well as difficult chronic fistula in this context.
Primary ischioanal abscesses are approached either as a bedside or formal operative procedure. Secondary ischioanal infections offer a unique opportunity for definitive management in which persistent and recurrent infection can be minimized. The surgical approach to the deep postanal space entails a crypt to coccyx midline incision. The anococcygeal ligament is sectioned vertically in its midline. A transsphincteric seton may be placed through the primary posterior fistulous trajectory.
The belief that supralevator abscesses are rare needs to be reconsidered. Supralevator abscesses were identified in one large series in 9.1% of cases. Therefore, while less common they comprise nearly one in ten cryptoglandular infections [13]. More than half have no obvious physical signs on visual inspection. Palpation of a boggy mass within the rectum is the diagnostic sine qua non. More than half of supralevator abscesses have the deep postanal space as their primary site of infection.
Intrarectal drainage of a type I intersphincteric supralevator abscess is definitive treatment. If the intersphincteric primary fistulous trajectory is identified, an internal sphincterotomy incorporating it may be performed. It may be preferable to place an intersphincteric seton in special cases including an anterior trajectory in a female or a posterior midline trajectory in either gender. The latter diminishes the risk of forming a posterior keyhole deformity that enables seepage of mucous subsequently (Fig. 1.4).


Fig. 1.4
Type I intersphincteric supralevator abscess. a Incorrect external drainage through the ischioanal fossa results in b a suprasphincteric fistula. c Correct internal drainage of the abscess with an intersphincteric seton shown. d Alternative internal drainage in continuity with an internal sphincterotomy demonstrated
Anterolateral type II extrasphincteric abscesses are drained external via the ischioanal fossa with or without a transsphincteric seton through the primary fistulous trajectory. Because of the depth of the drainage tract, most surgeons place a supralevator drainage catheter in the space. Once the space is collapsed several weeks later, this catheter is withdrawn. Some surgeons make no effort in localizing the primary fistulous trajectory thereby simplifying wound care with the single catheter drain (Fig. 1.5).


Fig. 1.5
Type II extrasphincteric supralevator abscess from primary transsphincteric ischioanal process. a Incorrect drainage internally can lead to b spontaneous decompression of the ischioanal fossa. This intervention may produce either an F-type fistula as depicted (c) or an extrasphincteric fistula (d). If the primary fistulous component is identified, a transsphincteric seton and drainage catheter are employed. e If not identified, only a drainage catheter is placed (f)
Posterior type III extrasphincteric abscesses are managed similarly to a deep postanal space infection with vertical sectioning of the anococcygeal ligament. A supralevator catheter may be placed in the setting of a large retrorectal abscess. A transsphincteric seton through the primary fistulous trajectory is also an option in this scenario (Fig. 1.6).


Fig. 1.6
Type III extrasphincteric supralevator abscess from a posterior midline process. a A posterior midline incision is used to explore the postanal spaces. The superficial postanal space is evaluated and then the anococcygeal ligament sectioned to enter the deep postanal space. Passage through the pubococcygeus muscles allows evacuation of the supralevator component. b A primary seton is placed through the internal opening if identified. Counter incisions can be made over the ischioanal fossa bilaterally if needed. A retrorectal catheter can be inserted to decompress the supralevator abscess
A type IV supralevator abscess has the deep postanal space as its primary focus of infection. There is a secondary extension of the deep postanal space infection to the ischioanal fossa. Both the deep postanal space and ischioanal infections contribute to the supralevator abscess simultaneously. Surgical treatment is directed at the primary site of infection in the deep postanal space with counter drainage of the involved ischioanal fossa (Fig. 1.7).
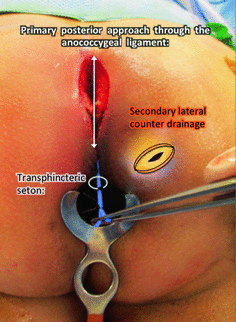

Fig. 1.7
Type IV extrasphincteric supralevator abscess from a primary posterior midline process with ischioanal component. A posterior exploration (crypt to coccyx) is required to evaluate the postanal and the supralevator spaces. The posterior process can be treated with catheter drainage with or without a seton. A counter incision is made to drain the ischioanal component externally. Catheter drainage may be implemented to address the supralevator component in this infection
Abscesses presenting as a distinct fullness on the posterior wall of the rectum also merit special consideration. Both posterior type I and type III abscesses should be considered. The key to this differential diagnosis is inclusion or exclusion of the deep postanal space as the primary infection (Fig. 1.8).


Fig. 1.8




Retrorectal supralevator (Type I or III). a These abscesses may have normal topography. b Fullness is encountered within the rectum posteriorly. c The ischioanal fossae are normal. This clinical scenario may represent either a type III configuration (d) or type I configuration (f). Computed tomography can assist in delineating the difference between type III (e) or type I (f) to guide drainage
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







