Organism
Uropathogens cultured
Percent that were urease-positive
Proteus spp.
54
100
Klebsiella spp.
31
84
Staphylococcus spp.
67
55
Escherichia coli
142
1.4
Pseudomonas spp.
20
5
Providencia spp.
1
100
Morganella morganii
1
100
Total
423
28.8
Urease catalyzes the hydrolysis of urea, a process often called ureolysis, producing two molecules of ammonia (NH3) and one molecule of inorganic carbon (Eq. 5.1). Ammonia is a Brønsted-Lowry base (i.e., a proton acceptor) and therefore generates hydroxide (OH−) ions that can increase the pH of urine from near neutral to levels as high as 9 [7]. The pH increase and the production of ammonium (NH4 +), an ionic constituent of struvite, promotes precipitation.
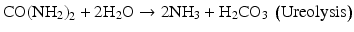
Implanted devices such as stents, catheters, pouches, and meshes increase the risk of UTI occurrence, but infections can also develop without a foreign device. It is likely that the migration of pathogens to the kidney is aided by surface-attached microbes, also known as biofilms. Biofilms produce extracellular polymeric substances (EPS), mostly polysaccharides, which provide protection against physical and chemical threats to bacteria. The role of biofilms in oral, skin and indwelling medical device infections has been documented for several decades [8], and biofilms play an important role in the colonization of the urinary tract and migration of infectious bacteria into the kidney. Upstream cellular motility, in conjunction with surface-adhering biofilms, likely allows pathogens to overcome peristaltic flow through the ureters and ultimately infect the kidneys [9] (Fig. 5.1).
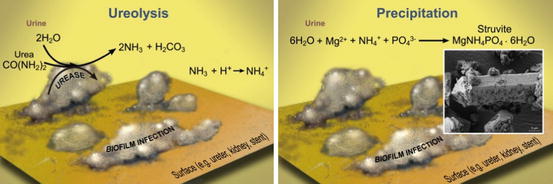
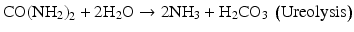
(5.1)

Fig. 5.1
Heterogeneous biofilms can develop on urinary tract surfaces. (left) If the biofilm contains microbes that produce urease, urea is hydrolyzed and the chemistry of urine is affected. (right) When the chemistry of urine is affected by ureolysis, struvite can precipitate. The inset image shows a struvite crystal that developed in a laboratory reactor cultivating a ureolytic biofilm (Trace Hobbs and Ellen Lauchnor, 2014, unpublished data)
In addition to protecting the bacteria, biofilms can play a role in struvite stone formation. At the interface of the biofilm and urine, cells are in direct contact with the substrates required for struvite formation (i.e., urea, Mg2+, PO4 3−), thus there is a close relationship between the ureolytic activity in the biofilm and stone formation. Furthermore, EPS can serve as a matrix glue to allow smaller crystals – not only struvite but also other stones such as calcium oxalate – to combine into larger stones [10].
For several reasons, UTIs can also promote the formation of metabolic stones, i.e. those that are not normally associated with infection [11]. For example, an increase in urine pH promotes the formation of calcium phosphate stones because it shifts the phosphate speciation from HPO4 2− to PO4 3−. Another example is that higher urine pH accelerates the breakdown of ascorbic acid into oxalate, thus promoting calcium oxalate stones [12].
Ureolytic Precipitation of Struvite
The precipitation of struvite (MgNH4PO4 · 6H2O), described in Eq. 5.2, requires dissolved ions of magnesium (Mg2+), ammonium (NH4 +) and phosphate (PO4 3−). Sterile urine contains dissolved Mg2+ and PO4 3−, and sufficient concentrations of NH4 + can be produced by bacterial ureolysis (according to Eq. 5.1). For precipitation to occur, the ion activity product (IAP) must be greater than the ion activity product at equilibrium in a solution with struvite (Ksp). The activity of a species is an effective concentration, which takes into account interactions with other species. Thus, in urine where other ions are present, they will interact with each other and change the “effective concentration” of neighboring ions. Because activity is based on a ratio comparing concentration to the pure species concentration, it is dimensionless by convention. In very dilute solutions, activity is well-approximated by concentration. If the IAP is greater than the Ksp, the saturation index (Eq. 5.3) is positive and the solution is called supersaturated. For struvite at body temperature, the Ksp value is approximately 10−13 [13]. Supersaturation implies that precipitation is possible, but we must consider that slow rates of precipitation or molecules that inhibit precipitation (e.g., proteins or polysaccharides that either block surfaces or sequester ions from solution) can allow a solution to remain supersaturated on practical time scales without precipitation. In other words, supersaturation is necessary for precipitation, but does not guarantee that it will occur.
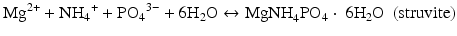
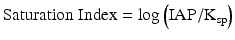
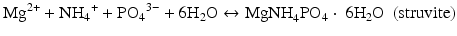
(5.2)
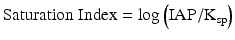
(5.3)
Example Problem
At a location in the urinary tract, the struvite ion activity product (IAP) is 10−12. If the Ksp of struvite at body temperature (37 °C) is 10−13, is the solution supersaturated? If struvite does not precipitate in this system what are some possible explanations?
ANSWERS: Yes, the solution is supersaturated. Precipitation could not occur because
(a)
There are inhibitors that sequester ions or lower their activity.
(b)
The kinetics are too slow relative to the residence time of the system.
The mechanism of struvite stone formation starts with nucleation and proceeds via layer-by-layer crystal growth along with the aggregation of crystals. The precise mechanisms of nucleation and growth are controlled by the saturation index, impurities, and surface structures. For a detailed description of crystal growth and nucleation, the interested reader is referred to De Yoreo and Vekilov [14]. Conceptually, it is important to keep in mind that, very likely, all nucleation in the urinary tract occurs on pre-existing surfaces (i.e., heterogeneous nucleation) rather than in solution (i.e., homogeneous nucleation). In other words, nearly all crystals will be associated with surfaces, but these surfaces could be from suspended particles in the bulk fluid (e.g., bacteria, proteins, dead human cells, etc.), and not only the surface of the ureter, catheter, etc.
Biofilms can promote the aggregation of small crystals to form larger stones. For this reason, infection stones are known to grow exceptionally fast compared to metabolic stones [15]. Furthermore, the shape and properties of struvite stones are influenced by the organic molecules that are incorporated within the stones, such as polysaccharides, mucoproteins, and glycosaminoglycans [16]. Treatment methods aimed at breaking up or dissolving infection stones must therefore consider the biomolecules integrated within the stones.
Techniques to Investigate Struvite Stone Formation In Vitro
Mineral precipitation and biofilm formation are governed by micro- and nano-scale processes that are difficult to observe in vivo. Until recently, our knowledge of stone formation and treatment has relied primarily on clinical observations such as treatment efficacy and patient history. But in order to understand the underlying processes, clinical-based hypotheses must be tested in controlled experiments. Laboratory (in vitro) systems and computer (in silico) simulations offer advantages for studying stone formation. Noninvasive clinical observations are generally limited to urine analysis and ultrasound, MRI or CT imaging which do not have the spatial and temporal resolution required to study microbe-induced stone formation in detail. Furthermore, ethical issues prevent many types of controlled in vivo experiments (e.g., varying pH, the addition of non-clinically-approved compounds or the use of destructive imaging techniques).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




