Stone Disease of the Urinary Tract
Nimalan Arumainayagam
Introduction
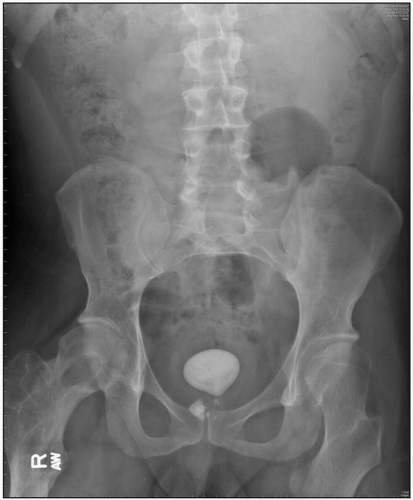
The first documented urinary tract stones were found among the 7000-year-old remains of the pelvic bones of a teenage boy in El-Amara. Before the Industrial Revolution in Britain, bladder stones were more common than upper urinary tract calculi. Later, around the turn of the nineteenth century, upper tract stone disease began to become more prevalent. In developing countries bladder calculi are still endemic. 4.1 shows the radiographic images of such bladder stones.
Stone disease in developed countries is more common in males, and in up to 80% of cases there is no obvious precipitating cause.
The presenting symptoms of upper urinary tract stones are loin pain, which may radiate to the groin in a colicky nature, and haematuria (microscopic or macroscopic). If urinary sepsis is present, there may also be symptoms and signs of infection. Stones in the bladder, and sometimes in the lower ureter, may cause lower urinary tract symptoms (frequency urgency and nocturia from their irritative effects, poor stream and hesitancy. Bladder calculi may also cause sudden changes in urinary stream due to the stone acting as a ‘ball-valve’ over the bladder outlet, and the symptom of ‘strangury’ where the bladder can contract strongly and painfully in an attempt to evacuate a calculus.
Stone formation
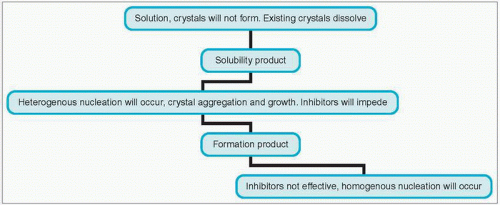
Stone formation occurs when elements normally dissolved in the urine form crystals that then aggregate (4.2). Stone formation may be either homogeneous (where the nucleus of the stone around which crystals aggregate is the same material as that of the crystal) or heterogeneous (where it is different).
Critical to the formation of any crystal is the saturation state of the solution. The saturation state of a solution depends on the pH and ionic strength of the given solution, as well as the presence of any soluble complexes.
When the solution is not saturated with a specific solute, then no crystals will form or grow, and any crystals already present will dissolve.
With increasing concentrations of solute, the solution will become fully saturated with that solute (saturation product KSP). If the concentration of the solute is increased even more, then the formation product (KFP) is reached.
Between the saturation product and the formation product, crystals of the solute cannot form spontaneously, but can aggregate on previously formed crystals (homogeneous crystallization), or aggregate on different formed crystals or foreign bodies (heterogeneous crystallization).
Once the concentration of the solute is greater than the formation product (KFP), the solution becomes unstable and spontaneous crystal formation occurs. Such crystallization can be inhibited by other constituents (e.g. magnesium and citrate are proven inhibitors of stone formation in urine).
Stasis contributes to stone formation and thus stones tend to form at sites of obstruction to urinary flow.
Risk factors for stone formation
There are specific risk factors that if present in an individual can lead to an increased incidence in the formation of certain types of stones (Table 4.1). There are a number of general factors that have been identified as rendering an individual more likely to make any of a number of types of stones which are summarized in Table 4.2.
Table 4.1 Risk factors for stone formation | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Table 4.2 Common types of stone | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Specific inhibitors of stone formation include magnesium, citrate, nephrocalcin, Tamm-Horsfall glycoprotein and uropontin.
Calcium stones
Calcium is the most common constituent of urinary tract calculi. Such stones are radio-opaque. Common states leading to calcium oxalate stone formation are listed in Table 4.3.
Sixty per cent of people with calcium stones will have an absorptive hypercalciuria, i.e. an altered intestinal response to vitamin D, leading to increased absorption of calcium. Ten per cent of patients with calcium stones will have renal hypercalciuria, i.e. the kidneys are unable to conserve calcium, and such patients develop hypercalcaemia (Table 4.4) and subsequent increased parathyroid secretion.
Hyperparathyroidism accounts for 6% of people with such urinary tract stone disease.
Type 1 renal tubular acidosis (autosomal dominant inheritance) usually leads to the formation of pure calcium phosphate stones. In this disease, patients are unable to acidify their urine below a pH of 6.0, and have decreased excretion of bicarbonate, potassium and citrate in the urine.
Table 4.3 Metabolic risk factors for calcium oxalate stone formation | |||||||
|---|---|---|---|---|---|---|---|
|
Hyperoxaluria may be either congenital or acquired. Primary hyperoxaluria (excessive production and hence urinary excretion of oxalic acid) is rare, and results in severe recurrent calcium oxalate stone formation leading to nephrocalcinosis. The most common type of acquired hyperoxaluria is enteric and occurs in malabsorption-type syndromes where the colonic epithelium is exposed to bile salts, resulting in increased permeability of the colon to oxalate. Dietary factors are important in all types of hyperoxaluria. Dietary calcium binds oxalate and prevents it from being absorbed from the gut. Large quantities of dietary calcium supplements are sometimes needed in the treatment of enteric hyperoxaluria.
Calcium oxalate. Also called whewellite or ‘mulberry’ stones, these stones are characteristically dark brown/black in colour, with a dense, smooth appearance (4.3A). 4.3B shows the crystals under electron microscopy. Calcium oxalate monohydrate crystals are dumbbell-shaped when viewed under light microscopy.
Table 4.4 Common causes of hypercalcaemia | |||||
|---|---|---|---|---|---|
|
Magnesium ammonium phosphate stones
These are also called triple phosphate or struvite stones, named after Heinrich von Struve who first described them.
They are usually formed in the presence of chronic urinary infection. Urea-splitting organisms within the urine (e.g. Proteus species) cause the urine to become more alkaline with its pH rising above 7.0, which causes precipitation and stone formation (Table 4.5).
Table 4.5 Common infectious organisms capable of struvite stone formation | |||||
|---|---|---|---|---|---|
|
This classically causes the formation of staghorn calculi within the renal pelvis and calyces. The classic staghorn calculus is composed of magnesium ammonium phosphate. They are usually yellow/white in colour and smooth in texture (4.4A). Note its appearance under electron microscopy (4.4B)
Struvite stone crystals are said to have a ‘coffin-lid’ appearance under light microscopy.
Uric acid stones
Humans are unable to convert uric acid to allantoin, and therefore uric acid levels in humans are much higher than in many other mammals. Pure uric acid stones are radiolucent and therefore are not seen on plain KUB (kidneys, ureters and bladder) radiographs.
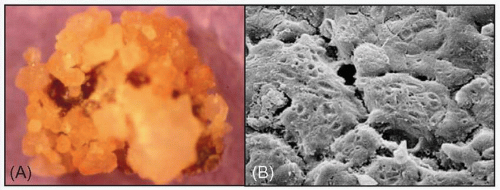 4.4 Magnesium ammonium phosphate stones shown macroscopically (A) and under the electron microscope (B). |
Uric acid is the end product of purine metabolism (Table 4.6




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree