Fig. 8.1
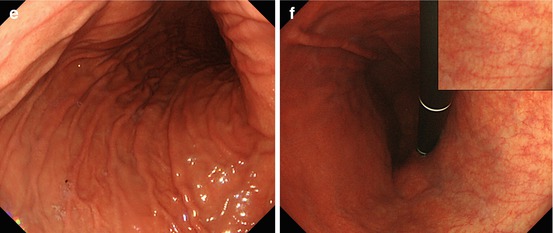
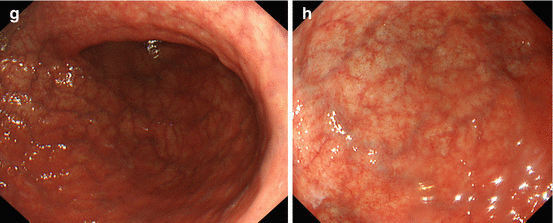
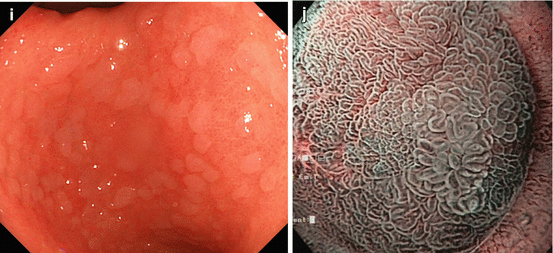
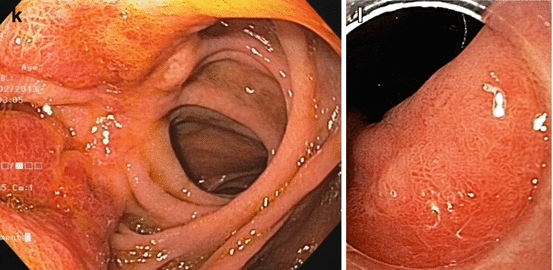
(a, b) Normal fundic gland mucosa extends in the area of gastric folds and (b) shows fine red dots on WLI (lesser curvature), i.e., starfish-like, regular arrangement of thicker collecting venules (RAC; insert 20× WLI), as a characteristic aspect of intact fundus/corpus mucosa free of chronic gastritis. (c) M-NBI (40×) shows well-defined roundish, slit-like pit pattern and regular network-like MC pattern for fundic-type mucosa, and (d) pyloric-type mucosa in antrum shows villous surface pattern with fine helix-like MCP and even white zone (WZ, i.e., crypt epithelium); insert 60× (e, f) Chronic atrophic gastritis displays (e, f) reduction and loss of gastric mucosal folds and (f) permeation of regular pattern of larger collecting submucosal venules on standard WLI endoscopy due to atrophy of mucosal glands (g, h) Chronic gastritis caused by Helicobacter pylori leads to typical changes with (g) loss of gastric folds and (h) loss of RAC pattern (most prominent on lesser curvature side) (i) Specialized intestinal metaplasia (SIM) in chronic gastritis exhibits slightly elevated whitish areas with uncertain margins on standard WLI. (j) Specialized intestinal metaplasia (SIM) in pyloric-type mucosa exhibits villous surface pattern with “light blue crests” (LBC = augmented WZ) and diminished regular spiral microvascular pattern on magnifying NBI (k) Chronic remnant gastritis 30 years after distal gastrectomy with B-II anastomosis. (l) Chronic gastritis at anastomosis with whitish IM, unclear margins (for M-NBI, see Fig. 8.1j)
Usually, gastric folds can be observed within only fundic gland area. Therefore, you can recognize fundic gland area by the gastric folds (Fig. 8.1a).
Sometimes, H. pylori infection causes atrophic gastritis, and gastric folds will disappear. Usually, the atrophy started from lesser curvature side. Therefore, you must observe the gastric folds of lesser curvature, when you insert scope into stomach. And, if you find folds in lesser curvature, the risk of gastric cancer is low (Fig. 8.1b). However, if the folds disappeared, that means atrophic gastritis (Fig. 8.1e–h), and the risk of gastric cancer is high.
The other important risk factor is H. pylori infection. Usually, you can observe red dots in the lesser curvature of gastric body (Fig. 8.1b). It is named regular arrangement of collecting venules (RAC) by K. Yagi [8]. If you can observe RAC, the risk of H. pylori infection is low – that means the risk of gastric cancer is also low. But if you can’t observe RAC pattern (Fig. 8.1h), that means H. pylori infection yielding higher risk of gastric cancer.
Key notes:
1.
Folds of gastric body
2.
RAC
Chronic atrophic gastritis. Intestinal metaplasia (IM) in chronic gastritis exhibits slightly elevated whitish areas with uncertain margins on standard WLI, and surface pattern with “light blue crests” on M-NBI (Fig. 8.1i–l) [9]. Typical such patterns predict the histological findings with approximately 90 % accuracy.
8.2.2 Basic Endoscopic Structure of Early Gastric Neoplasia
Gastric adenomas usually present as [7, 10, 11] (Fig. 8.4):
Protruded (0-Is;-Isp) or elevated (0-IIa) lesions
More pale aspect on WLI
Nodular pattern with indigo carmine spreading
Regular villous pattern by magnified NBI (M-NBI)
Differentiated adenocarcinomas [7, 11, 12] (Figs. 8.5d and 8.6)
Form all type-0 lesions (Is, IIa/b/c, III) with clear margins
Appear reddish
Present irregular surface pattern
Irregular pit or villous pattern by M-NBI
Fine microvascular network pattern by M-NBI
Undifferentiated gastric cancers of small size show up [7, 12, 15] (Fig. 8.8):
Mostly as 0-IIc and 0-IIb lesions.
As pale color on WLI endoscopy.
Well demarcated in fundic gland area. But, with uncertain margin in atrophic area.
Surface pattern is uncertain; microvessels are corkscrew-like by M-NBI.
8.3 Observation with Conventional WLI Endoscopy
On WLI endoscopy, any lesion is defined by color, macroscopic type, and lateral margins. Reddish color reflects permeation of augmented mucosal and submucosal microvessels. The first distinction is between protuberant (Is) and flat lesions (IIa, IIb, IIc).
8.3.1 Differential Diagnosis of Protuberant Lesions on WLI
Protuberant lesion, when reddish and with uncertain margin, most likely is an inflammatory lesion (Fig. 8.3a, b). Clear margins of a red protuberant lesion strongly support hyperplastic polyp (nonneoplastic) (Fig. 8.3c, d) or well-differentiated adenocarcinoma (algorithm in Fig. 8.2a). Diagnosis is made using biopsy and M-NBI, because hyperplastic polyp shows regular surface and vascular patterns (Fig. 8.3c, b).
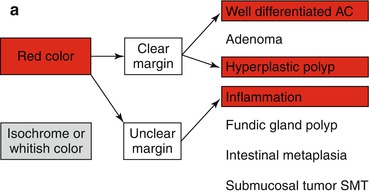
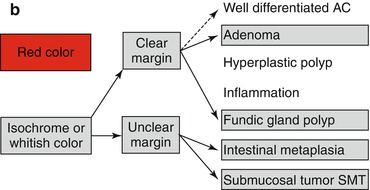
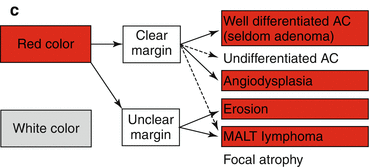
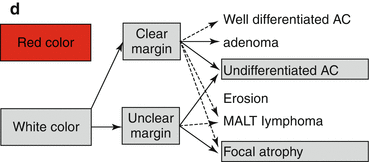
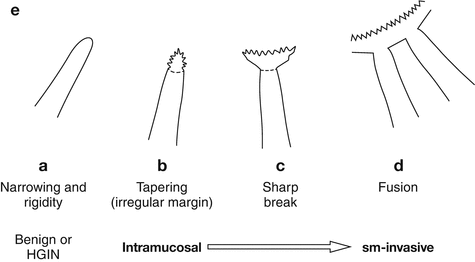
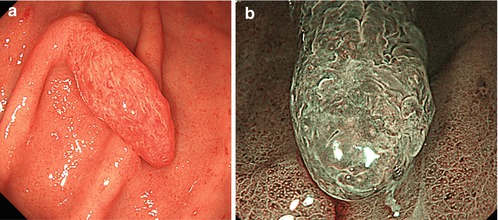
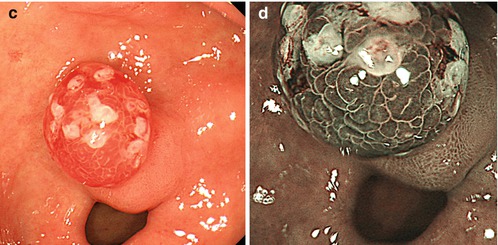
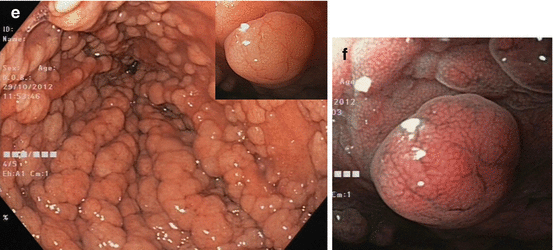
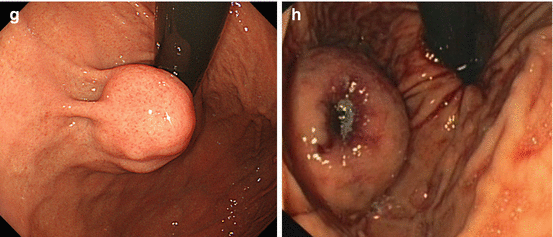





Fig. 8.2a-e
(a) Differential diagnosis of reddish protuberant gastric lesions (b) Differential diagnosis of whitish (isochrome) protuberant gastric lesion type 0-Is or 0-IIa on standard WLI endoscopy (c) Differential diagnosis of reddish depressed lesions type 0-IIc (d) Differential diagnosis of pale, whitish flat, or depressed lesions type 0-IIc (e) (a–d) Mucosal folds at flat depressed or ulcerated gastric lesions (0-IIc, III). Information on likely vertical extension of early gastric AC gained from shape of folds: (a) at benign ulcer or intramucosal AC (EP, LPM), (b) intramucosal AC (EP, LPM), (c) slightly sm-invasive AC (MM, sm1), (d) deeply sm-invasive AC (≥sm2)(Modified from [16])




Fig. 8.3
Inflammatory polyp 0-Ip in fundic gland corpus (a) WLI, (b) magnifying NBI (40×) (c) Hyperplastic polyp (reddish on WLI) (d) on NBI (20×), distinct pyloric-type glands (e) Multiple fundic gland polyps, FAP patient, WLI (insert 20×). (f) Fundic gland polyp, NBI (20×) (g) Submucosal gastric tumor – bridging folds and regular fundic-type mucosa. (h) Submucosal gastrointestinal stromal tumor (GIST) with mucosal invasion and ulceration (diagnosis by EUS and biopsy from ulcer)
Whitish protuberant lesion with clear margins supports the diagnosis of fundic gland polyp (Fig. 8.3e, f), adenoma (Fig. 8.4a–k), or less likely well-differentiated adenocarcinoma (WDAC) (Fig. 8.6b–d). Whitish or isochrome protuberant lesions with uncertain margin most likely represent intestinal metaplasia (Fig. 8.1i–l) or submucosal tumor (Fig. 8.3g, h) – compare algorithm in Fig. 8.2b [16].
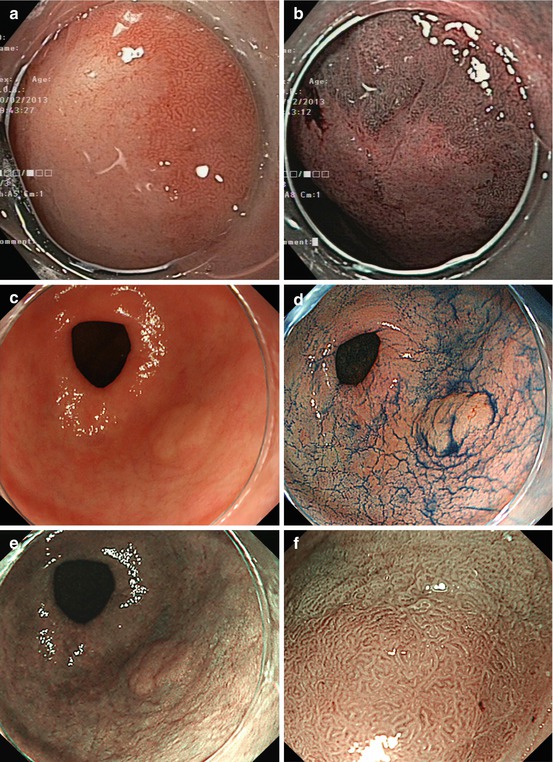
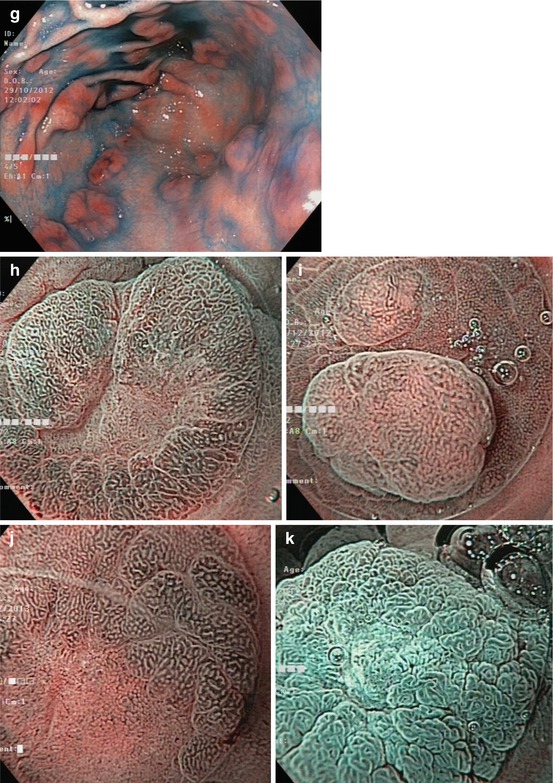


Fig. 8.4
Pyloric-type gastric adenomas. (a) Clear margin of flat whitish adenoma (0-IIb) vs. pyloric-type mucosa on WLI and (b) distinct margin of pale adenoma to pyloric-type mucosa with chronic gastritis on NBI. (c) Pale adenoma (0-IIa) in chronic gastritis (pyloric-type mucosa), (d) indigo carmine-acetic acid CE, (e) standard NBI, (f) villous surface and even white opaque zones of adenoma (top) with clear margin to antrum mucosa (bottom) with SIM (40× magnifying NBI) Patient with FAP (g) multiple adenomas (0-Is, 0-IIa, 0-IIa + c) in antrum, WLI with indigo carmine CE; (h) Lesion 0-IIa + c, micrified villous mucosa (gastric adenoma), clear margin, and hyperplastic mucosal rim (pyloric-type mucosa), M-NBI (60×, under water). (i) Protruding lesion 0-IIa with irregular pit/ridge pattern (small gastric adenoma) within fundic-type gastric mucosa (pits). (j) Micrified fundic pit pattern with clear margin of gastric adenoma and (k) surface enhancement after application of acetic acid [same as (j)]
8.3.2 Differential Diagnosis of Depressed Lesions on WLI Endoscopy
Reddish depressed lesions with clear margins more likely reveal well-differentiated adenocarcinoma (WDAC) (Figs. 8.5d and 8.6d, g) or rarely angiodysplasia (Fig. 8.5a, b). Red lesions with uncertain margins usually are erosions or seldom MALT lymphoma (Fig. 8.5e, f) (very seldom WDAC or adenoma) (Fig. 8.2c).
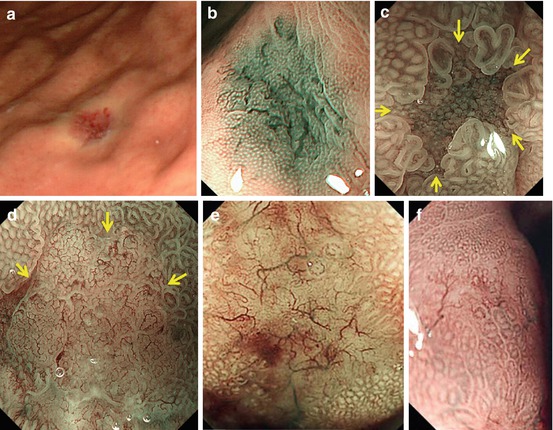
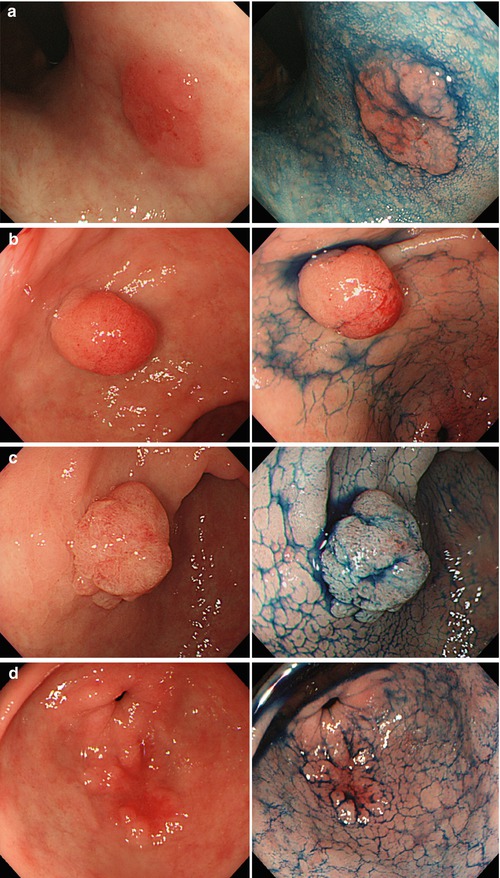
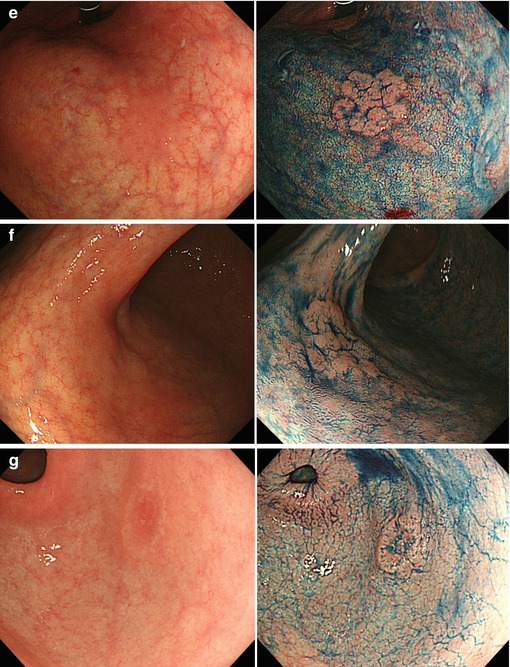

Fig. 8.5
(a, b) Angiodysplasia in gastric corpus, ordinary WLI, and M-NBI (40×). (c, d) Depressed lesions 0–IIc (3–3.2 mm), M-NBI 100×, demarcation line (arrows). (c) Focal atrophy in chronic gastritis with IM showing regular surface pattern (pit or villi) and vascular pattern (open-loop). (d) Depressed differentiated early gastric cancer with clear margin, uncertain surface and irregular microvascular patterns (From [13] with permission by John Wiley and Sons/Digestive Endoscopy). (e) Mucosal lymphoma showing disappearance of surface structure, unclear margins, and tree-like appearance of abnormal blood vessels (M-NBI, 80×) in gastric corpus [WLI: multiple slightly reddish lesions 0-IIa and 0-Is; biopsy: mantle cell lymphoma]. (f) Same, in partial remission after six courses of chemotherapy (Permission granted by Thieme/Endoscopy [14])


Fig. 8.6
(a–d) Signs for intramucosal extension of differentiated AC. Intramucosal extension of cancer (adenocarcinoma AC) is common in distinct homogeneous protuberant lesions (a) type 0-IIa, (b) type 0-Is smoothly homogenous, (c) 0-Is smoothly lobulated, and (d) reddish depressed-type 0-IIc. Left panel, standard WLI; right panel, acetic acid-indigo carmine CE (AIM) (e–g) Flat types of well–differentiated gastric adenocarcinoma (WDAC). Standard WLI (left) shows reddish lesions, indigo carmine CE (right) reveals macroscopic type and lateral margins of WDAC: (e) type 0-IIa, (f) type 0-IIb, (g) type 0-IIc. Types IIa and IIb have low probability of submucosal invasion; type IIc carries substantial risk of sm invasion (in particular when >2 cm). M-NBI analysis of microvascular pattern is mandatory for flat types of early gastric cancer
Pale (whitish) flat or depressed lesions with clear margins are highly suspicious of gastric AC, typically undifferentiated AC (Fig. 8.8), rarely well-differentiated AC (Fig. 8.7a–e), or adenoma, but may reveal focal atrophy (Fig. 8.5c) or MALT lymphoma on histology. Pale depressed lesions with unclear margins may represent focal atrophy, rarely undifferentiated AC (Fig. 8.8a–d), or MALT lymphoma [13, 16] (algorithm Fig. 8.2d).
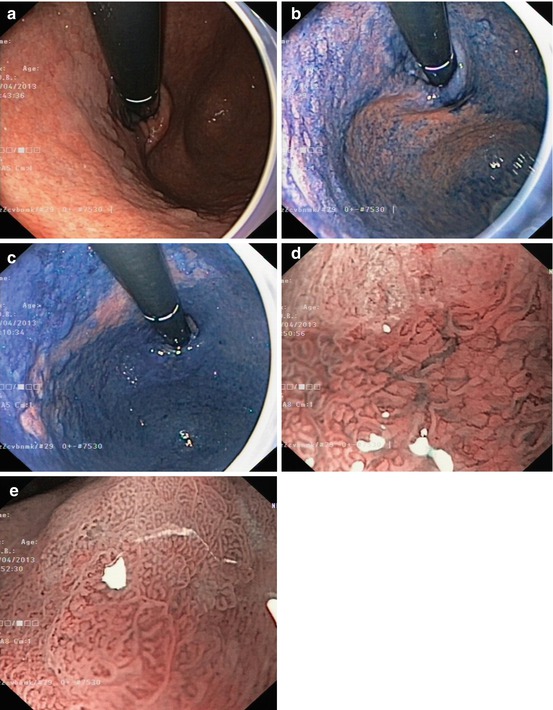
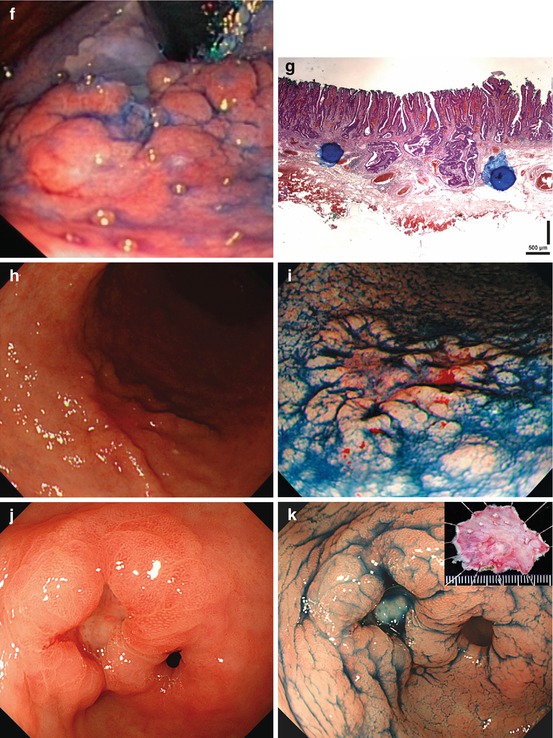
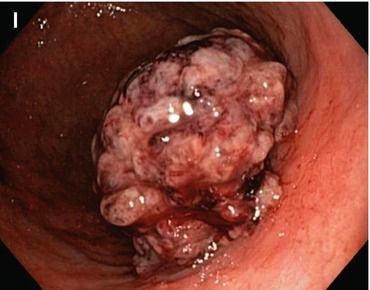
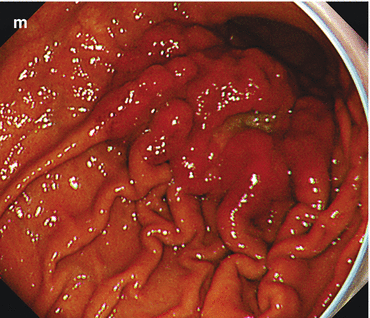
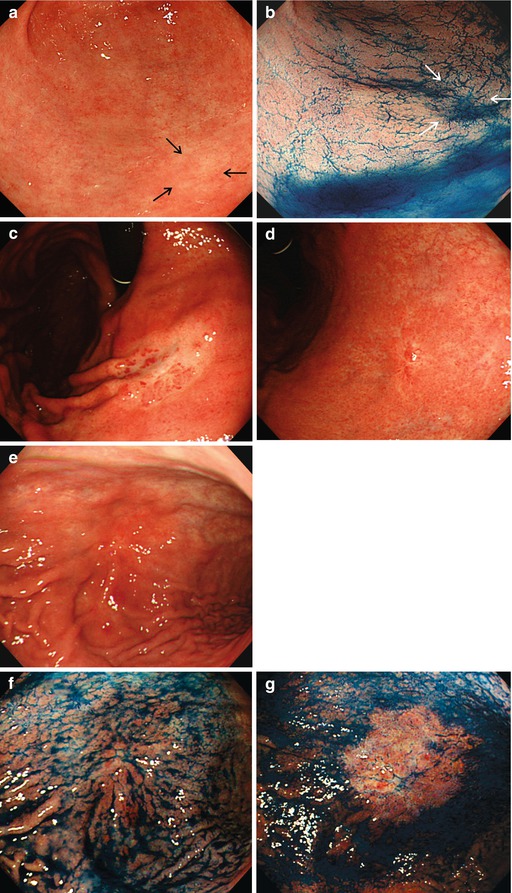








Fig. 8.7
(a–e) Signs for intramucosal extension of differentiated AC. Intramucosal extension of cancer (WDAC, HGIN) is common in distinct homogeneous lesions type 0-IIb. (a) Pale lesion 0-IIb in chronic Helicobacter-induced gastritis, WLI, (b) indigo carmine CE only, (c) acetic acid-indigo carmine CE (AIM), (d) center with irregular CP; (e) clear margin (left, HGIN). ESD en bloc: WDAC (G1 pT0m1), resection R 0 Note: pale lesion 0-IIb (HGIN) in chronic Helicobacter-induced gastritis: Lateral extension is obscured by indigo carmine only (b) but revealed by AIM-CE (c). Magnifying NBI shows regular SP, network CP (d, e), and clear margin of the neoplasia (e) (f–k) Indirect signs of massive submucosal invasion of differentiated AC: (f) depression in 0-IIa + IIc lesion at gastric cardia ((g) AC G2. 560 μm sm2-invasion, HE stain 100-fold) (h, i) Elevation with loss of regular surface structure (amorphous pit pattern) in 0-IIc cancer (j, k) ulcer in depressed 0-IIc lesion (0-IIc + III ) ((j) WLI; (k) IC-CE). Similar significance as (f) conveys ulcer in sessile 0-Is lesion with amorphous surface structure (l) Indirect signs of massive submucosal invasion (probability >80 %) of differentiated AC: (l) irregular protuberance, expansive nodular growth, “fullness of the stalk” in 0-Is or 0-IIa (m) Infiltrating undifferentiated adenocarcinoma often shows thick and irregular folds , some folds with fusion in the center of the lesion, an alteration highly suspicious for massive sm invasion (compare Fig. 8.3g for noninvasive submucosal tumor)

Fig. 8.8
(a–g) Poorly differentiated early gastric cancer PDAC type 0-IIb in pyloric-type mucosa. (a) PDAC lesion 0-IIb (arrows) on standard WLI, (b) indigo carmine CE, white arrows mark the lesion. (c, d) Typical presentation of PDAC in fundic gland mucosa. Whitish depressed lesion 0-IIc with clear margin in fundic gland area. WLI in (c) slight insufflation, (d) full insufflation of stomach. (e–g) Poorly differentiated early gastric cancer PDAC type 0-IIb + c. Isochrome-whitish depressed lesion 0-IIb + c in gastric major curvature with break and some fusion of folds, typical for sm-invasive PDAC in chronic gastritis. (e) Standard WLI, (f) indigo carmine only rather obscures, (g) AIM truly reveals lateral extension of cancer
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








