Bladder neck contracture is a relatively uncommon but well-described complication after the surgical treatment of prostate cancer. Although numerous treatments have been described as an initial management strategy for patients with this condition, the management of refractory cases remains highly variable. This article evaluates various therapeutic maneuvers used for the treatment of refractory bladder neck contracture and further describes the preliminary results of an endoscopic balloon dilation with concomitant deep traunsurethral incision procedure. Short- and long-term management algorithms for patients with recurrent bladder neck contractures are reviewed.
Key points
- •
BNC is associated with surgical factors during prostatectomy, smoking history, diabetes, coronary vascular disease, and pelvic radiation.
- •
Urethral stenting has high rate of complication and need for reoperation.
- •
Urethral dilation and endosopic incision are successful in the great majority of patients with BNC.
- •
Transurethral injection therapy is a promising option for refractory BNC.
- •
Open reconstruction remains a viable option for refractory BNC when endoscopic management fails.
- •
Management of stress urinary incontinence after BNC treatment can be safely performed after stability of bladder neck is determined.
Introduction
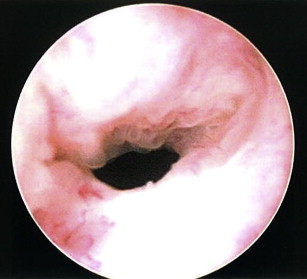
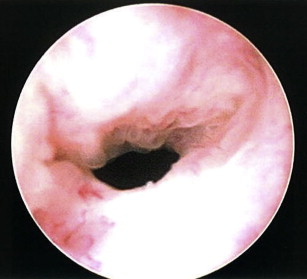
With the number of patients treated by radical prostatectomy (RP) or radiation for localized prostate cancer on the rise, the development of bladder neck contracture (BNC; Fig. 1 ) after treatment remains a recognized complication treated by urologists in contemporary practice today. Although the incidence of BNC after RP has continued to decrease over the last 20 years with the advent of robotic surgery and refinements in surgical technique, a small subset of patients nevertheless develop anastomotic strictures warranting treatment. Many patients undergoing initial treatment of BNC achieve successful early results, but a significant group of patients experience recurrent obstruction. As a result, patients with refractory BNC pose a difficult clinical management scenario for urologists. Although recent efforts have focused on determining factors associated with the occurrence of BNC to better direct prevention and treatment, the management of refractory disease remains challenging and unstandardized. Most contractures are often managed successfully with conservative measures, such as serial dilation or transurethral incision, with acceptable success rates ranging from 50% to 87%.
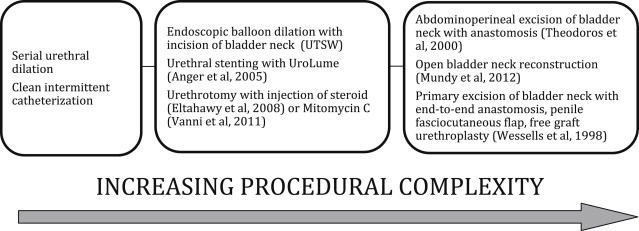
When conservative approaches fail, patients with refractory BNC require additional treatments with increasing procedural complexity ( Fig. 2 ). As such, patients with refractory and recurrent BNC present a challenging management dilemma that is the focus of this article. Here we summarize various methods used to manage patients with refractory BNC at our institution.
Introduction
With the number of patients treated by radical prostatectomy (RP) or radiation for localized prostate cancer on the rise, the development of bladder neck contracture (BNC; Fig. 1 ) after treatment remains a recognized complication treated by urologists in contemporary practice today. Although the incidence of BNC after RP has continued to decrease over the last 20 years with the advent of robotic surgery and refinements in surgical technique, a small subset of patients nevertheless develop anastomotic strictures warranting treatment. Many patients undergoing initial treatment of BNC achieve successful early results, but a significant group of patients experience recurrent obstruction. As a result, patients with refractory BNC pose a difficult clinical management scenario for urologists. Although recent efforts have focused on determining factors associated with the occurrence of BNC to better direct prevention and treatment, the management of refractory disease remains challenging and unstandardized. Most contractures are often managed successfully with conservative measures, such as serial dilation or transurethral incision, with acceptable success rates ranging from 50% to 87%.
When conservative approaches fail, patients with refractory BNC require additional treatments with increasing procedural complexity ( Fig. 2 ). As such, patients with refractory and recurrent BNC present a challenging management dilemma that is the focus of this article. Here we summarize various methods used to manage patients with refractory BNC at our institution.
Background: causes and incidence of BNC with associated risk factors
The primary treatment sustained by the patient often plays a central role in the cause of BNC development. In patients undergoing pelvic radiation therapy for malignancy, microvascular effects and progressive obliterative endarteritis leading to tissue necrosis of the bladder neck is a well-described phenomenon. Conversely, in patients undergoing RP, it is often suggested that BNC arises because of technical factors surrounding surgery. Prior reports have suggested that most BNC can be prevented during RP in particular through a tension-free watertight anastomosis and mucosal eversion at the time of surgery. Thus, it is not surprising that the incidence of BNC has decreased after robotic-assisted laparoscopic prostatectomy (0%–3%) when compared with historical data after open RP (0.5%–32%). BNC is also a well-known complication of endoscopic treatment of benign prostatic hyperplasia. Although conventional transurethral resection of the prostate was noted to have a BNC rate between 1% and 12.3%, a smaller rate of BNC has been noted with newer technologies, such as the KTP laser prostatectomy (3%–5%).
Many proposed factors contribute to the development of BNC after prostate surgery, including surgical technique, surgeon experience, postoperative complications, and patient comorbidities ( Box 1 ). In a retrospective analysis of 467 patients undergoing RP, Borboroglu and colleagues studied the development of BNC in 52 patients. Through a multivariable analysis assessing for predictors of BNC, the authors discovered that intraoperative blood loss, increased operative time, a positive smoking history, diabetes mellitus, and the presence of coronary artery disease were all significant predictors linked to the development of BNC. In our institution, we have also noted a significant correlation between a greater than 10 pack per year smoking history and the development of recurrent BNC when compared with nonsmokers.
Coronary artery disease
Hypertension
Diabetes mellitus type 2
Current smoking history
Open radical prostatectomy
Body mass index ≥30
Age ≥60
Age
Charlson score
Body mass index
Renal insufficiency
Postoperative urinoma
Postoperative hematoma
A recent review of the Cancer of the Prostate Strategic Urologic Research Endeavor database revealed that patient age, body mass index, and primary type of treatment were all prognostic indicators for the development of clinically significant strictures requiring treatment in patients undergoing primary treatment of localized prostate cancer. This analysis also demonstrated that although BNC after prostatectomy typically presents within 6 months after surgery, patients treated with primary radiation often present years after treatment likely because of progressive radiation-induced fibrosis and necrosis. After multimodal radiotherapy (eg, brachytherapy plus external-beam radiotherapy or salvage prostatectomy), even higher rates of BNC have been reported with devastating consequences to the bladder outlet and patient quality of life.
Urethral stent placement
The UroLume (American Medical Systems, Minnetonka, MN) was introduced in 1988 by Milroy as a novel, minimally invasive stent to manage urethral stricture disease. Although initial studies regarding the use of the UroLume were promising, numerous problems were encountered with urethral stenting, including stent migration, obstruction secondary to tissue in-growth, hematuria, stent encrustation, and the need for repeat surgery.
Stent migration, in particular, had been reported in 23% of cases and additional studies had demonstrated that the onset of stent migration manifested anywhere between 6 weeks and 3 years after initial UroLume placement. Although a 100% success rate was noted in the eight patients initially studied by Milroy and colleagues, a subsequent report by Hussain and colleagues demonstrated a complication rate of 50% in 60 patients that underwent urethral stenting. De Vocht and colleagues also reported concerning rates of persistent urinary incontinence (50%) and a notable proportion of patients (27%) undergoing surgical revision secondary to stent obstruction.
Although initial efforts to define the role of UroLume placement focused on the management of urethral stricture disease and detrusor-sphincter dyssynergia, a recent report by Erickson and colleagues evaluated the application of the UroLume specifically in patients who had undergone primary radiation or surgery for the treatment of prostate cancer. In a series that included 38 men, the study investigators reported an initial success rate of 47% with 53% (N = 19) of patients requiring repeat intervention for stent complications. As such, because of the frequency of postprocedural complications and need for further surgical treatment, urethral stenting is now rarely indicated. At our institution, the use of the UroLume stent has been abandoned because patients with bladder outlet obstruction secondary to BNC have been managed with acceptable rates of success through endoscopic balloon dilation and incisional procedures.
Urethral dilation and endoscopic incision
Initial management of patients with BNC has conventionally centered on conservative measures in lieu of invasive surgical maneuvers. Intermittent dilation of the bladder neck has therefore been used to treat contractures and prevent recurrent disease. If the lumen of the contracture is amenable to urethral catheterization, gradual dilation may be performed in a clinical setting or with the use of an outpatient serial dilation program. As such, several prior works have assessed the efficacy of urethral dilation in the management of BNC after RP. In a large series (N = 510) of postprostatectomy patients in the United Kingdom, a BNC rate of 9.4% (N = 48) occurred with all patients successfully managed through urethral dilation or clean intermittent catheterization. Similarly, Park and colleagues used a regimen of urethral dilation to 18F catheter followed by a 3-month period of intermittent self-catheterization in 32 postprostatectomy patients with BNC. With at least 1 year of follow-up, 93% (N = 24) of patients were successfully treated with one or two dilations followed by self-catheterization. Although self-catheterization provides a nonsurgical approach to BNC management, significant patient tolerance and compliance seem to be necessary for successful results. Additionally, some patients experience complications of self-dilation, such as the development of urinary tract infection, hematuria, false passage, and urethral stricture.
An alternative technique with low procedural morbidity involves a cold-knife endoscopic incision of the bladder neck for patients with BNC. Although numerous studies have evaluated the efficacy of cold-knife urethrotomy in first-time diagnoses of BNC, most reports have limited patient follow-up and do not report long-term results of patients undergoing multiple endoscopic treatments for recurrent stenosis. In a large series (N = 52) of BNC undergoing intervention, 22 patients (42%) required at least one repeat dilation or transurethral incision, whereas six patients (11.5%) required more than two therapeutic interventions. Thus, when repeat dilation or endoscopic therapy for recurrent BNCs is necessary, subsequent success rates are inferior to those of initial transurethral incision or dilation.
Taking into account the known risks of repeat endoscopic therapy in patients with highly refractory disease, a hybrid endoscopic procedure incorporating both balloon dilation and incision has recently been developed at our institution. A brief description of this endoscopic technique is included next.
Surgical Approach and Procedure
Instruments that should be available to the surgeon during transurethral incision of a recurrent BNC are listed in Box 2 . A 3% sorbitol solution should be used for irrigation. The case is started with careful examination of the entire urethra with a flexible cystoscope or 21F catheter rigid cystoscope. A 0.083-in Amplantz Super Stiff Guide Wire is passed through the BNC into the bladder ( Fig. 3 ). With the wire in place, a 4-cm, 24F catheter UroMax Ultra High Pressure dilating urethral balloon (Boston Scientific, Natick, MA, USA) is advanced over the Super Stiff Guide Wire (Boston Scientific, Natick, MA, USA) through the BNC. Balloon dilation of the urethra is performed under direct vision through a 21F catheter rigid cystoscope or alongside a 16F catheter flexible cystoscope to verify that the dilating balloon is engaged within the contracture. Dilation to a pressure level of 15 atm is performed with the use of the LeVeen pressure syringe ( Fig. 4 ) under direct cystoscopic vision to ensure that the balloon does not migrate during active dilation. The balloon is then left in place dilated across the area of stenosis for approximately 3 minutes to allow scar tissue around the area of contracture to conform to a round configuration. After this is complete, the balloon and the wire may be removed after cystoscopy is performed to ensure no synchronous bladder lesions or stones are present.
Monopolar energy source
3% sorbitol irrigation solution
21F catheter rigid cystoscope
0.083-in Amplantz Super Stiff Guide wire
4-cm, 24F catheter UroMax dilating balloon
16F catheter flexible cystoscope
LeVeen pressure syringe
24F catheter resectoscope
Collings knife
22F catheter two-way soft Foley catheter
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







