Fig. 45.1
MRI showing circumferential resection margin (see red line)
Conversely, MRI is excellent in identifying the CRM [9]. Accuracy for MRI depends on understanding the fields of alignment. Inappropriate technique can lead to under- or over-staging of tumors. Correct field alignment is made in relation to the long axis of the rectum. T2-weighted images are most suitable for distinguishing the layers of the bowel wall [10]. The different layers of the bowel wall are identified from their unique signal characteristic. Disruption through the normal signal characteristic pattern determines the depth of invasion. The depth of spread into the mesorectum is of particular importance. This fatty layer which surrounds the rectum in varying degrees along its length acts as an oncological barrier to tumor spread. Tumor spread which invades the mesorectum is classified as T3. The majority of patients present with T3 tumors, however there is wide variety in survival rates of these patients [11]. Cawthorn reported 5 year survival to be 55 % with tumor penetration less than 4 mm into the mesorectum compared to 25 % when more than 4 mm [12]. Merkel studied patient’s survival characteristics with T3 tumors and used a cut-off of 5 mm. Those patients with extramural spread of more than 5 mm had 5 years survival rate of 54 % compared with 85 % for those patients whose tumors had extramural spread of less than 5 mm [13] (Fig. 45.2).
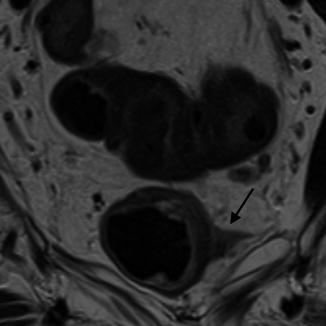

Fig. 45.2
MRI showing tumor penetrating into mesorectum (see black arrow)
These early studies highlight the importance of accurate measurement of tumor penetration into the mesorectum and those tumors with a worse prognosis, namely T3c and T3d. Therefore the distinction between T2 and T3 tumors with less than 5 mm mesorectal spread—T3a and T3b; becomes irrelevant as these patients will have minimal benefit from CRT [3]. The accuracy of MRI in delineating the mesorectal fascia and producing comparable results to histological analysis has been shown by the MERCURY Study Group [14, 15]. Two hundred and ninety-five patients who had undergone primary surgery had imaging and histopathological analysis of tumor depth compared. There was correlation between MR and histopathological assessment of tumor spread to within 0.5 mm.
Nodal Disease
Knowledge of tumor spread to locoregional lymph nodes forms part of the local staging of rectal cancer and is an integral component of the AJCC TNM classification [1]. Local lymph node involvement is a known independent marker of poor prognosis [16, 17]. Tumors which exhibit nodal disease independent of depth of tumor penetration (Stage III and Stage IV) are more likely to be offered pre-operative radiotherapy although the rationale behind this is becoming less convincing. There is now clear randomized trial evidence that optimal surgical technique is far more important than pre-operative radiotherapy in reducing the risk of local disease recurrence [3, 18].
Accurate identification of malignant lymph nodes has been traditionally challenging. As imaging modalities become more accurate in their ability to define adverse features, detailed information on tumor characteristics are expected and influence pre-operative treatment decisions far more. The “gold-standard” for lymph node disease remains histopathological analysis of the resection specimen. However this is only influential in post-surgical setting. Lymph node architecture can be identified on MR. Figure 45.3 demonstrates the anatomical features which can be seen on high resolution images.
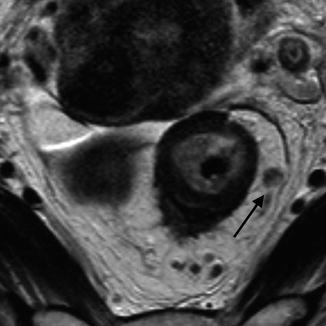

Fig. 45.3
MRI showing a heterogeneous, irregular lymph node in the mesorectum (black arrow)
Traditionally, lymph nodes size has been a marker of suspicion of malignant disease. This is not the case and has led to incorrect overstaging of nodal disease based solely on size criteria. Although MRI has difficulty in picking up lymph nodes less than 3 mm, however less than 2 % of these are thought to be malignant [19]. In terms of size, and this is maximum diameter, 5 mm may exclude a significant proportion of malignant nodes [19]. In 2003, a study meticulously matching nodes from the in vivo and specimen MRIs with pathology specimens to undertake node for node analysis showed that there was no useful size cut-off for predicting nodal status [19]. This was well supported by existing histological evidence from Dworak—a histological survey of over 12,000 lymph nodes in rectal cancer showed considerable size overlap between normal or reactive nodes and those containing metastases [20].
The outline or border of the node and the signal characteristics demonstrated on MR are important determinants in whether it is benign or malignant. The outline or border of the node alone may help differentiate benign from malignant nodes. A smooth, regular outline is seen in benign nodes. A very small number of lymph nodes with smooth border contour (<6 %) have been shown to be malignant whilst those demonstrating irregular outline are malignant in over 90 % of cases [19]. This characteristic shows the highest levels of sensitivity and specificity. However, when using the signal characteristics and border outline together, the sensitivity is much improved. Signal characteristic is a better and more accurate method of detecting malignant nodes. A universal hypo- or hyper-dense signal is less likely to indicate malignant node involvement compared to a mixed signal characteristic. These nodes show remarkable correlation with histological analysis of nodes. The mixed signal on MR is shown to be areas of necrosis.
EAUS does not predict lymph node involvement any better. Sensitivity and specificity for detection of cancerous lymph nodes in rectal cancer is 73.2 and 75.8 %, respectively [21] although more likely to be accurate in the more proximal parts of the rectum. Swollen reactive nodes, small blood vessels and even local structure such as the seminal vesicles may mimic malignant nodes.
In addition to nodes within the mesorectal envelope, nodes on the lateral pelvic sidewall must be considered. There are differing approaches to how these nodes should be managed between the East and West. Japanese surgeons have traditionally offered patients a more radical operation which involves clearance of these lateral nodes. The argument against this approach is that a significant proportion of patients who will undergo an extensive surgical procedure for little benefit. These nodes are offered neo-adjuvant CRT in the West. It is difficult to visualize these nodes on EAUS in contrast with MRI which is the optimal modality for detection [22]. Studies investigating the identification of lateral wall lymph nodes are almost exclusively limited to Japan. In almost 80 % of cases, the presence of lateral nodes is associated with malignant mesorectal nodes. The incidence of malignant lateral lymph nodes ranges between 8.6 and 24 %, though these rates are mainly from advanced, and often low rectal cancers [23–25] with most studies reporting rates around 10 %. Identification of sidewall lymph nodes remains important as it is a marker for other prognostic factors such as increased mesorectal nodal burden and extramural venous invasion [22]. A mechanism of spread to the pelvic sidewall may well be through the extramural vasculature. Indeed one small randomized trial suggested that CRT and pelvic sidewall dissection resulted in equivalent oncological outcomes [26].
Circumferential Resection Margin
One of the main advantages of MRI over EAUS is that it can identify the mesorectal fascia that forms the radial margins of excision—the circumferential resection margin (CRM) [9]. Local recurrence of rectal cancer following surgical resection has been dramatically reduced by the introduction of total mesorectal excision (TME). MRI has been shown to accurately predict the relationship between tumor and mesorectal fascia with a high degree of concordance with histology. There is now overwhelming evidence that the tumor spread to involve the CRM is the single-most important determinant of pelvic recurrence and this is not compensated for by the use of radiotherapy [3, 18] (Fig. 45.4).
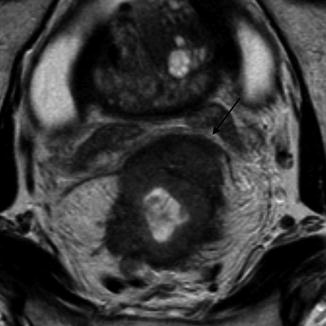

Fig. 45.4
MRI showing tumor at the circumferential resection margin (CRM) anteriorly (black arrow)
Tumor spread which threatens the potential CRM has been shown to be a predictive factor of local recurrence [27]. It is now generally accepted that tumor spread within 1 mm of the potential CRM is a strong influence in local recurrence. In fact, one may consider that tumor spread to the CRM is most probably the most important factor in local failure. Recent work by Taylor et al. has shown that rates of local recurrence decreased from 53 % with tumor less than 1 mm from the potential CRM to less than 8 % when the tumor distance from the mesorectal fascia was between 1 and 5 mm [28].
Being able to accurately identify tumor proximity to the mesorectal fascia within a millimeter on MRI has been a challenge. A measured distance of 5 mm on MRI has been shown to strongly correlate with negative CRM on histology, which led to patients being offered chemoradiotherapy when tumors are within 5 mm of the mesorectal fascia. However, this results in substantial overt-treatment of patients with safe margins. The MERCURY Study Group reported that margins could be identified more accurately and reproducibly, using a 1 mm cut-off [28].
Whilst tumor spread to the circumferential margin is well documented to increase the risk of local recurrence [27, 29–31], there is still some confusion regarding the presence of lymph nodes lying close the mesorectal fascia. One suggestion has been that if a lymph node containing tumor cells lies within 1 mm of the CRM thus being staged as a pathologically involved margin, the nodal capsule acts as a barrier to spread and does not lead to tumor recurrence [32]. Shihab et al. analyzed patients from the MERCURY study where there were suspicious nodes within 1 mm of the CRM on MRI. None of these patients had an involved CRM. Thus nodes that are detected by MRI to lie within close proximity of the CRM are unlikely to increase the risk of tumor recurrence [33] (Fig. 45.5).
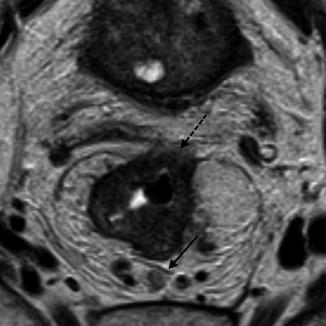

Fig. 45.5
MRI showing a lymph node at the mesorectal fascia. The solid black arrow shows the lymph node at the CRM posteriorly and the dashed black arrow shows the tumor at the CRM anteriorly
Extramural Vascular Invasion
Extramural venous invasion is defined as the presence of tumor cells in the vasculature beyond the muscularis propria. It is known to be associated with poor prognosis and increased rates of local recurrence and distant metastases [34]. It is seen in up to 50 % of rectal cancer patients and almost exclusively associated with more advanced tumors—T3 and T4. EMVI can be identified pre-operatively on MRI with great accuracy [35]. A recent randomized trial comparing neoadjuvant radiotherapy 45Gy plus capecitabine to radiotherapy 50 Gy plus capecitabine and oxaliplatin in patients with resectable T3–4 rectal cancer reported vascular invasion as an independent predictive factor of positive CRM [36] (Fig. 45.6).
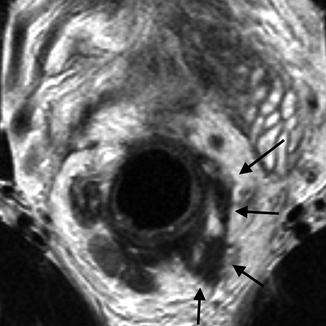

Fig. 45.6
MRI showing characteristics of EMVI, the black arrows shows the EMVI as a serpiginous expanded tumor deposit along the path of a vein
Using high spatial resolution MRI, veins are identified as serpiginous or tortuous linear structures on T2-weighted images. Assessment of EMVI using MRI must consider the following components: pattern of tumor margin which gives the appearance of nodularity; location of tumor to relevant vessels which makes tumor invasion more likely; caliber of vessel as tumor infiltration can cause an increase in luminal size; and vessel border if the tumor disrupts the vessel itself. Extension of the primary tumor into a vascular structure indicates EMVI [37].
EUAS is not able to identify this novel prognostic feature. Although the layers of the bowel wall and thus extramural depth may be identified, the spindle-like nature of the vessels and the subtle change in signal is not readily detected. This morphological feature plays more of a role in decision making in the UK and parts of Europe however future reports may lead to increased recognition of EMVI as an important prognostic indicator.
Low Rectal Cancer
Tumors that are defined as “low rectal cancers” have an inherent challenge. This is most likely due to the anatomy of these tumors located in the rigid confines of the pelvis. Traditionally, these tumors were most commonly surgically treated by abdominoperineal resection (APR) compared to anterior resection. There is evidence to show that local recurrence rates for low tumors treated with APR is much higher than low anterior resection [38–41]. The Low Rectal Cancer Study was borne out of the need to adequately stage low rectal cancers with MRI to define the surgical planes with accuracy with the aim to reduce the rate of positive circumferential resection margins in APR. Using high resolution MRI, a route map for low tumors is based in anatomical landmarks dividing the rectum into supralevator, intralevator, and infralevator sections. This allows surgeons to make operative decisions accurately based on MRI-predicted planes of excision [42]. A small retrospective study of 33 patients in which MRI was used to predict the plane of excision in order to achieve a negative resection margin reported a positive predictive value of 57 % and negative predictive value of 96 % [43]. Although these results are encouraging there must be appropriate training and education to standardize reporting of these tumors (Fig. 45.7).
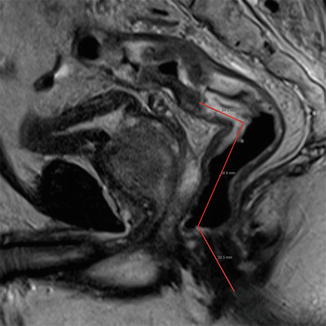

Fig. 45.7
Sagittal section MRI showing tumor height in relation to anal verge
45.3 PET/CT
Positron emission tomography (PET) has gained increasing popularity in the last few years. Although it has had limited uses for more than a decade, the combination of PET with CT has shown to be a particularly useful tool. The principle behind PET is the use of radioisotopes with a half-life of around 100 min that through tracers quantify pathological biochemical processes. These biochemical processes are thought to precede physical changes in the anatomy which makes this an exciting field. The most common tracer is fluorine-18-labeled deoxyglucose (FDG). This radioisotope acts as an analog to glucose and identifies cells which have an increased glucose metabolism such as cancer cells (Fig. 45.8).
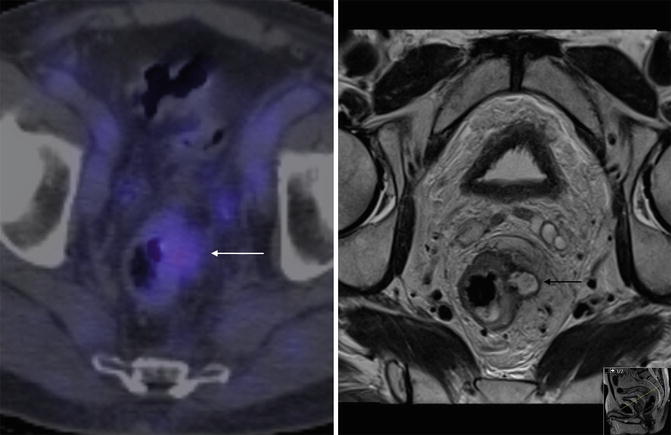

Fig. 45.8
PET/CT of rectum. PET/CT and T2 weighted axial MRI of the rectum in the same patient with recurrent rectal cancer. The white arrow shows ill defined uptake in the region of recurrent disease seen clearly on the MRI (black arrow)
The role of PET/CT as a combination in staging of rectal cancer is not by any means routine at present. However, it has been shown to have improved accuracy over CT and PET as individual examinations [44]. There remain several questions about the use of PET/CT and where it fits into the repertoire of imaging modalities in the staging of rectal cancer. In the context of pre-operative staging, there is little evidence to support its routine use [45]. No direct comparisons have been made in terms of accuracy of pre-operative local staging with MRI in large numbers and it is difficult to evaluate the sensitivity and specificity of this technique. However a recent study has shown that MRI and PET/CT may be used in combination for high risk patients (EMVI positive; extramural spread of >5 mm; T4 disease or an involved CRM) as part of a more intensive staging process [46]. In this study, patients were stratified by MRI into whether they were at high or low risk of developing synchronous metastatic disease. Almost 21 % of patients in the high risk group were confirmed to have metastatic disease on PET/CT compared to 4 % in the low risk group. These patients may benefit from a further PET/CT (or liver MRI) in the initial staging process.
Recent interest has been using PET/CT to assess response to neo-adjuvant chemoradiotherapy [47, 48]. Several small studies have been published within the last few years and have indicated positive results in terms of a predictive tool for tumor regression although one of the largest studies of this type has found no benefit in serial scans to assess response and prognosis [49–52]. The general methodology of these studies has been to perform PET/CT before and varying times after neo-adjuvant CRT and measured the response in terms of FDG uptake. Further work is necessary before this can be confidently used as a routine modality to assess tumor response. It may be too early to draw comparisons with an established technique like MRI, however the rationale behind using PET/CT as an imaging modality which can not only demonstrate anatomical change but also functional change, is certainly complimentary at a minimum.
45.4 Staging Metastatic Disease
Adequate local staging of disease is mandatory for surgical planning and treatment of the primary tumor. However, it is equally important to assess potential metastatic spread. This may influence the management of the primary tumor in terms of type and timing of treatment. The common sites of metastases of rectal cancer are the liver and lung. This may be related to the site of tumor within the rectum which has a variable lymphatic drainage as well as the influence of venous invasion on tumor behavior. For example, low rectal cancer is more likely to drain to the lateral pelvic sidewall than a tumor of the upper rectum. This lymphatic pathway differs from that of the more common route along the inferior mesenteric vessels. One may expect a different pattern of metastatic spread depending on the location of the tumor within the rectum.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








